Flow Cytometry Troubleshooting
|
Low SignalLow or no signals from your antibody staining, characterized as no signal above the isotype control or unstained control, can be due to a number of different factors depending on your experiment. View the categories to the left to explore possibilities for low signals.  |
Storage and Handling
Make sure your antibodies are kept at 4°C (between 2°C and 8°C) and shielded from light exposure. Do not freeze antibodies conjugated to fluors, as this can cause precipitation or aggregation of the antibody. You should also make sure the product has not expired.

Dilution
Are you using the recommended dosage per test? You may need to titrate your antibody to find the optimal amount to use for your unique experiment. Check your data sheet or CoA.
Filter Setup
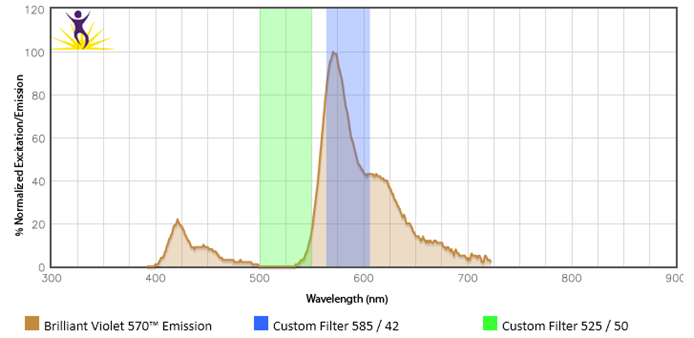
Be sure that your flow machine is equipped with the proper filters and lasers for the fluors you are interested in using. For example, Brilliant Violet™ 570 requires a violet laser and an optimal filter of 585/42. But, if someone tried to use the 525/50 filter they already have installed to detect BV570™, this would not work.
Fluors give off distinct emission profiles and the proper filters must be equipped in order to capture these wavelengths. In this instance, a 525/50 filter would catch wavelengths in the 500-550nm range. The peak emission of BV570™ (570nm) falls outside of this range and is not detectable by this filter.
Be aware of how many PMTs (Photomultiplier Tubes) each laser can handle. If you only have 3 PMT’s on the violet laser, but want to run 4 colors off it, this is not possible.
Laser Performance
If the lasers in your flow cytometer are not well-aligned, this will cause a weak signal in some (or all) channels. It is easy to check for instrument performance using our Rainbow Calibration Particles. Beads will fluoresce in every channel, so if you don’t detect signal in one of the channels, this is an indication that the cytometer needs to be checked. Please follow the cytometer manufacturer's instructions for quality control checks and optimal performance.
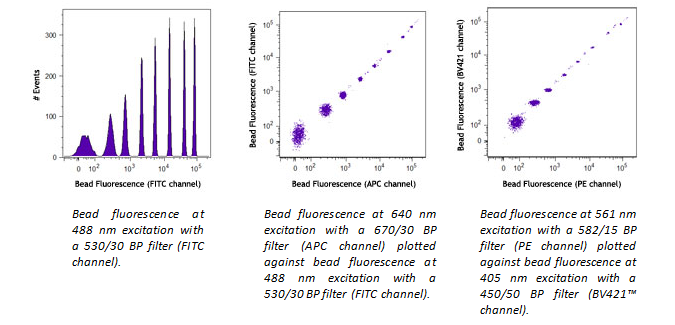
Light can scatter from the 532nm laser going into the FITC filter off the 488nm laser. The same is true with scattered 561nm laser light into the PE filter off when using the 561 nm laser. Use of a notch filter in front of the filters for those PMTs can reduce the background caused by the laser light scatter, maintaining sensitivity in the PMT.
Density
Certain markers are either expressed at low levels or on rare cell populations. In these instances, you should choose antibodies conjugated to the brightest available fluors:
Fluorophore Brightness Index
Cell Specificity
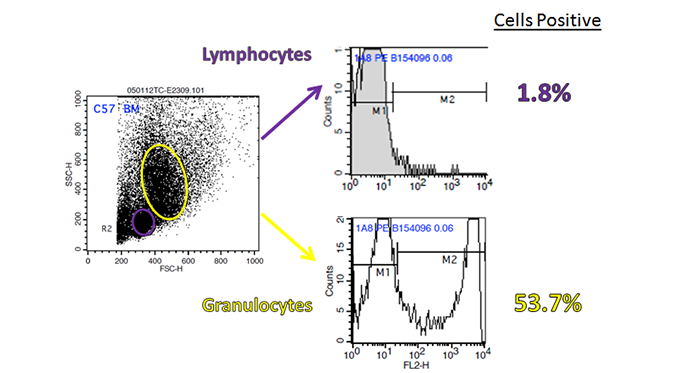
Certain haplotype-specific markers are only expressed by certain strains of mice, so carefully read the description before purchase. For example, H-2K, I-A, CD90, and CD45 for mouse, come in two or more characterized types, of which, each can be distinguished by haplotype-specific antibodies. In addition, as flow cytometry is based on hierarchal gating, an initial wrong turn in gate setting can have a negative downstream impact on data analysis. Be sure that you are examining the proper population before setting your initial gate. For example, if you are trying to examine Ly-6G on mouse bone marrow cells, it can readily be found within the granulocyte population, but not the lymphoid one. You may also want to make sure you’re examining the proper type of tissue/organ for the expression of your marker.
Location
Be aware that some proteins can be expressed on the surface of the cell, in the cytoplasm, internal organelles, the nucleus, or in multiple compartments simultaneously. Be sure to follow the proper protocol for their detection.
Induction
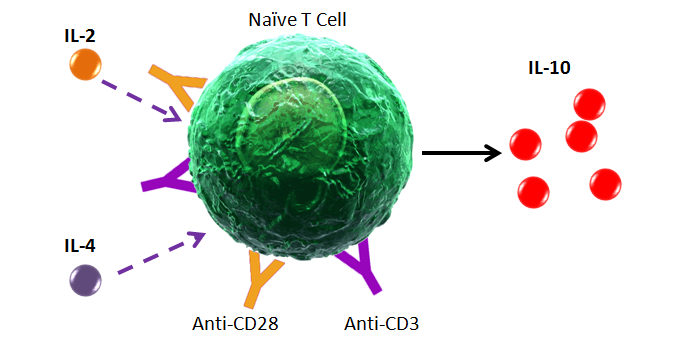
Some markers are downregulated from the cell surface upon activation. Other markers and cytokines must be induced in order to be detectable by flow cytometry. The type, concentration, and duration of stimulation can all have an effect on how robust the expression levels can be. For cytokines, secretion should be blocked using Monensin or Brefeldin A. This prevents cytokines from being secreted into the environment.
Intracellular Cytokine Stimulation Guide
Buffers
Are the proper buffers being used for your experiments? Our Foxp3 antibodies are optimized for use with our True-Nuclear™ Transcription Factor Buffer Set and may not work adequately with buffers from other vendors. In addition, certain flow cytometry applications require specific buffers for staining. The correct fixation and permeabilization buffers should also be used for intracellular cytokine staining. See our link below to examine our flow cytometry buffers in greater detail:
Flow Cytometry Buffers

Secondary Reagents
If you are using purified or biotinylated antibodies, are the corresponding secondary antibodies correct? For example, if the primary antibody is an Rat Anti-Mouse, be sure to use a secondary reagent like Goat Anti-Rat. Using a tube with only the secondary antibody allows you to assess the level of background staining in your product. You may also want to test whether your secondary antibody is working with other primary antibodies. This would help determine whether the issue is with the primary or secondary antibody.
Secondary Reagents
Fixation
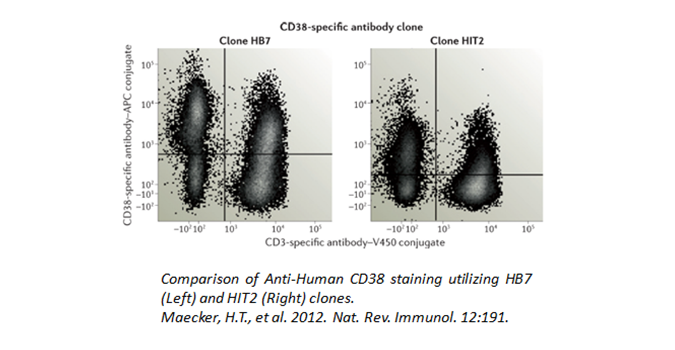
Are you fixing or permeabilizing your samples prior to staining? Fixation and permeabilization protocols can alter the interaction between epitope and antibody. This sensitivity is antigen-dependent and clone-dependent. For example, epitopes for clones like HIT2 (human CD38), 5C3 (human CD40), and BC96 (human CD25) are all particularly unstable with detergent or methanol treatments. In addition, some markers require a particular fixation for accessibility by antibodies, such as nuclear antigens. For instance, PCNA and Ki67 need ethanol fixation for its antibodies to bind properly. Your samples should never be exposed to fixation reagents for extended periods of time, as this can cause cross-linking which may affect the dye chemistry or increase autofluorescence.
Fixation
Adherent Cells and Receptors
If you are working with adherent cells and use trypsin to detach them, be aware that trypsinization could potentially affect the expression of surface molecules. A similar phenomenon can occur if the cells are treated with collagenase prior to staining. If you experience a loss of signal, you may want to try a gentler method to retrieve your cells.
Receptors may be internalized during the staining. Always stain at 4°C and use ice-cold reagents, if possible. Receptors can also be internalized if the cells have been stimulated in vitro prior to staining. Adding sodium azide to your FACS buffer may help to prevent receptor internalization.

Noise and Background
Having high noise and background in your PMT might also be interpreted as low signal. This can be due to several factors such as scattered light from the laser source, spillover from other fluorophores, or excessive autofluorescence.
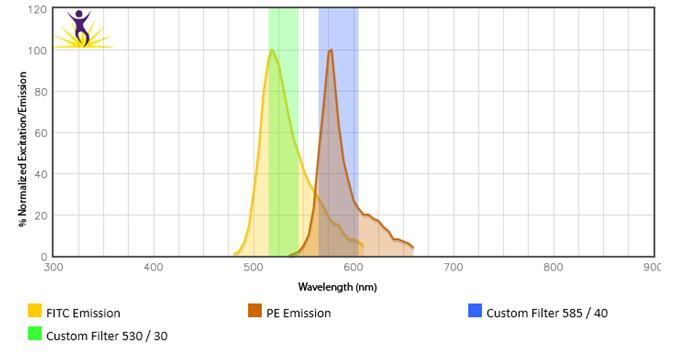
Spillover
Spillover is the leading cause of loss of sensitivity in any particular PMT when performing multicolor flow cytometry. Appropriate compensation must be applied to eliminate the spillover signal from neighboring fluorophores or cross-beam excited fluorophores. If the percentages of that compensation are high, there is a good chance that sensitivity in that PMT will be reduced.
|
High BackgroundHigh background, interpreted as non-specific staining, can be caused by a variety of factors, such as the fluorophore, the antibody, the protocol, the instruments, other reagents, or the data analysis. Explore a broad range of categories to the left that contribute to high background in your staining. |
Autofluorescence
Different cell types and tissues, as well as media additives, have varying levels of inherent fluorescence. Major sources of autofluorescence (AF) include NADH, riboflavins, flavin coenzyme molecules, crosslinking of primary amines by paraformaldehyde, and certain biological structures (e.g., mitochondria, lysosomes). Myeloid cell lineages tend to be particularly problematic with AF. These proteins and molecules are more easily excited at lower wavelengths (i.e. from the UV, violet, and blue lasers) and will emit at a wide range of 500-700 nm, which overlaps several common fluors like FITC and PE. You should always include a unstained control to check for the level of AF.
In addition, histograms may hide an autoflorescent population. In the example below, a bivariate comparison of FL-1 (GFP) and FL-2 for a GFP-expressing cell line reveals an autofluorescent population that can be gated out.
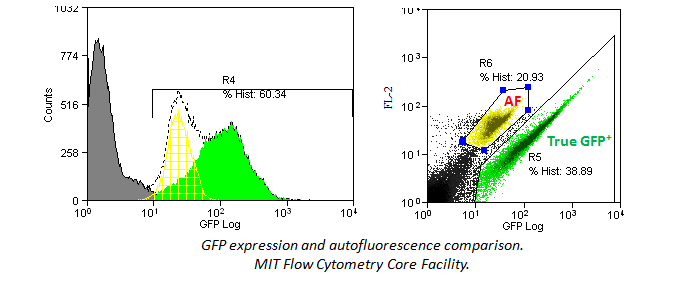
Dilution
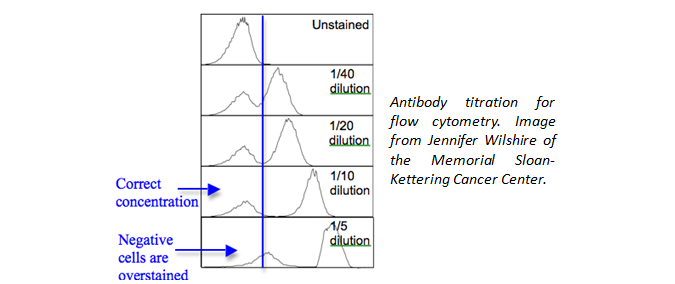
Is the proper amount of antibody being used? Although we have recommended amounts to be used, you could also titrate the antibody down to find an optimal amount to use for your unique experiment. This will help to eliminate background staining and improve your signal to noise ratio.
High Compensation
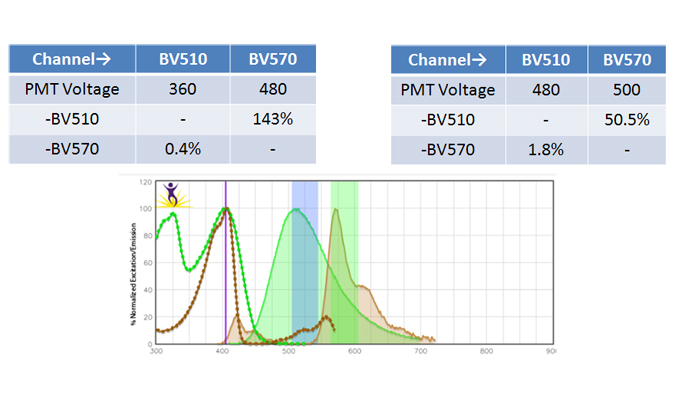
When two or more fluorophores overlap in emission spectra, some compensation will need to be applied. Depending on the amount of spillover, potentially high levels of compensation may be needed. In these cases, voltages that are being used for each PMT need to be balanced, because excessive differences in the voltage can amplify the requirement for compensation. For instance, BV510™ and BV570™ are known to require compensation because BV510™ will spillover into the BV570™ channel and vice versa. If the BV570™ channel’s voltage is much higher than the BV510™ voltage, this will amplify the spillover. Notice in the example below, when the PMTs are balanced the requirement for compensation is greatly reduced. If the appropriate levels of compensation are not applied, your background may appear artificially high.
Metabolic Activation
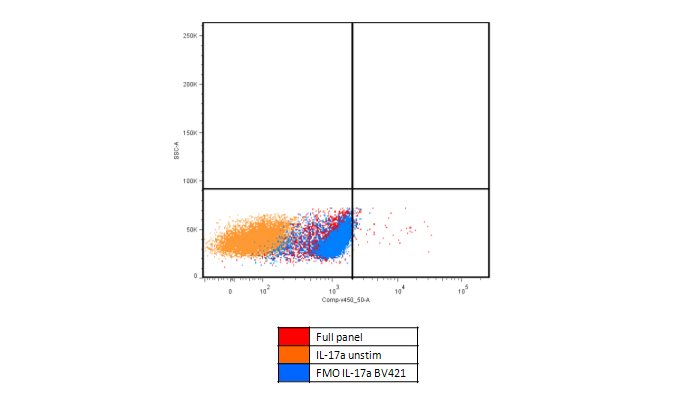
Increased background can be caused by metabolic activation. For example, when staining for IL-17A, in PMA activated PBMCs, the FMO control clearly shows an increase in the baseline in the BV421™ channel. This can be caused by several factors, including increased autofluorescence or increased spillover from other fluorophores.
Cyanine Dyes
Cyanine dyes and some of their tandems have a high propensity for binding to Fc Receptors, particularly monocytes. This reaction cannot be prevented with the use of a Fc Blocking reagent and its exact cause is unknown. If you are studying a population particularly high in myeloid-type cells (i.e., bone marrow) , you may want to consider using other fluors to denote your populations. Or, take a look at our True-Stain Monocyte Blocker™, which is specifically desgined to stop this interaction.
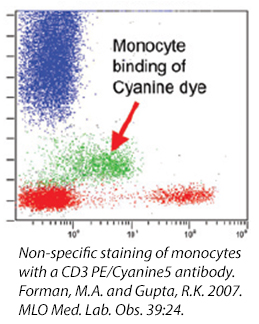
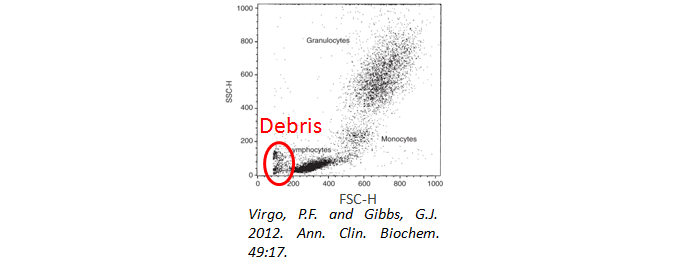
Cell Debris
Have you used a viability dye to ensure your cells are alive and happy during analysis? Cell debris and dead cells can lead to high background staining. In addition, when gating your cells, be sure to exclude the debris from your analysis. They will often have a very low forward and side scatter, as seen below.
Viability
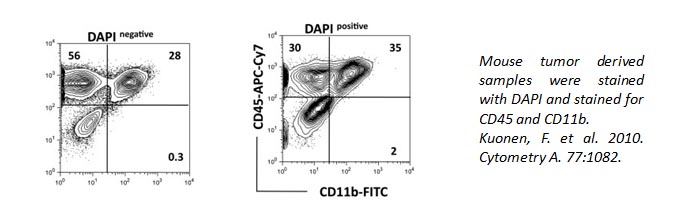
If dead cells/debris were to be accidentally included in your analysis, you may see false positive populations. Staining with Propidium Iodide or 7-AAD, which only enter into dead cells, is an excellent method to identify and exclude dead cells from your analysis.
DAPI can also be used to identify live/dead cells. It can also pass through live cell membranes, but in a less efficient manner. Below, the staining of CD11b and CD45 is altered within the DAPI+ population of cells compared to DAPI- cells.
For samples that need to be fixed and permeabilized (particularly with ethanol or methanol), DNA intercalating dyes like propidium iodide and 7-AAD are often not suitable. This is because the DNA can be unwound, popping the dyes off and allowing them to non-specifically bind cells that are now permeabilized. For these experiments, we would highly recommend Zombie Dyes, which bind to primary amines instead of DNA.
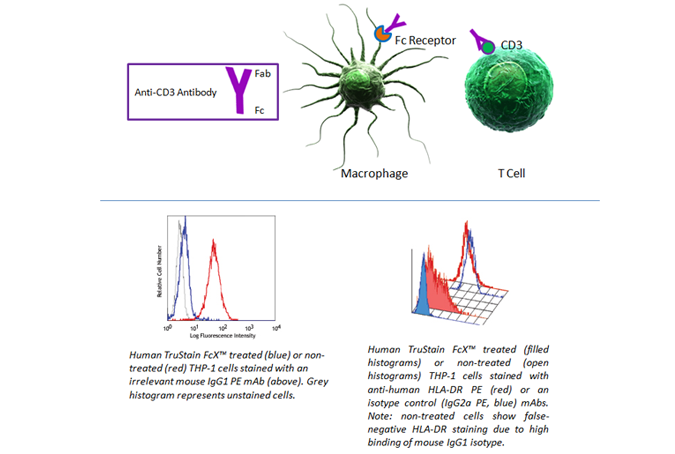
Fc Blocking
Fc receptors are present on many cell types, including granulocytes, B cells, macrophages, and dendritic cells. As they have a propensity to bind the Fc portion of antibodies, they can present false positives in your analysis. To prevent this background staining, we recommend using Human TruStain FcX™ (Fc Receptor Blocking Solution) (Cat. No. 422301) or TruStain FcX™ PLUS (anti-mouse CD16/32) Antibody (Cat. No. 156604).
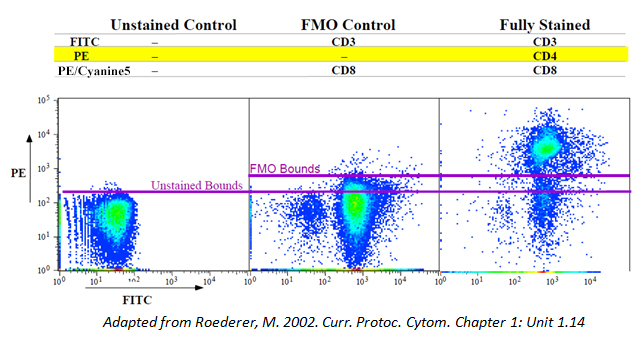
FMOs/Isotype Controls
Isotype controls are negative controls designed to help indicate the non-specific background staining (due to Fc Receptor or cellular protein interaction) of your test antibody. FMOs, or Fluorescence Minus One controls, can also be helpful, particularly if your antigen of interest is lowly expressed or you have a complex multicolor (4 or more) panel. This will help you observe any spillover from the other fluors in your panel and aid your selection of a true positive population.
You should also make sure to use the same amount of test and isotype control antibody. If you used 1 ug of Anti-CD4, use 1 ug of its corresponding isotype control.
Avoid purchasing isotypes and test antibodies from different companies, as the fluorophore:protein (F:P) ratio may not be consistent between different manufacturers.
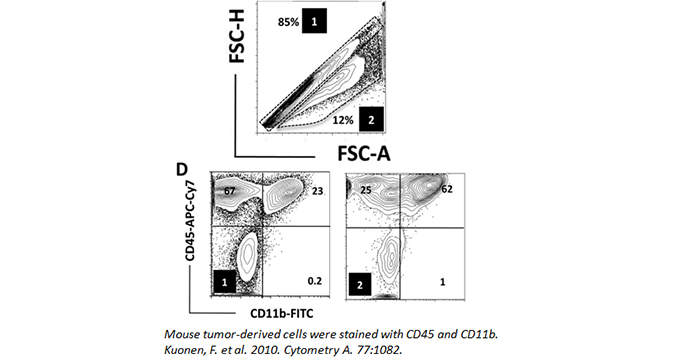
Doublets
Doublets can occur when cells clump together or are in the process of dividing. This presents difficulties when analyzing flow cytometry, as the cell mass will still count as one event. As such, if you do not gate them out of your analysis, they can lead you to misleading data. While it may seem like a double positive population may be present in your analysis, you should make sure it is not the result of two merged cells, with each cell expressing only a single marker.
Doublets can be minimized by adding EDTA to your FACS buffer and filtering your samples through a 30 micron filter. Doublets can also be easily gated out by assessing cell width, height, and area (as seen below). In the data, (1) denotes the singlet population, while (2) marks the doublets in the sample. The inclusion of doublets in your analysis can skew your results.
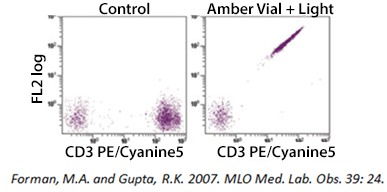
Tandem Degradation
If you are using a tandem dye, breakdown can occur if the antibody is not stored optimally and protected from light exposure. In the example below, a PE-Cyanine5 dye has been exposed to light. As the tandem breaks apart, a signal can be detected in the FL2 (PE) channel. Tandem dyes are also more sensitive to certain fixation methods. We recommend FluoroFix™ Buffer for fixation of samples using tandem dyes. You can learn more about tandem dyes from the link below:
Tandem Dyes
|
MiscellaneousIn addition to low signal or high background, there may be other difficulties researchers experience when performing flow cytometry. View these miscellaneous categories to the left. |
Cross Reactivity
Have you checked the compatibility of the product with your sample? An antibody that is known to react against mouse may not perform similarly with rat samples. A literature search could reveal if the clone’s cross-reactivity has been investigated. In addition, a homology comparison on Uniprot may give you a starting point to determine possible cross-reactivity. It should be noted, however, that even if an antigen displayed high homology between two species, this does not guarantee a cross-reactivity. BioLegend’s antibody cross-reactivity chart may also be a useful tool for you.
UniProt
Cross-Reactivity Chart
Fluorophores
Do you find that the PE format of an antibody from a competitor work better than the FITC format you obtained from BioLegend? Comparisons between fluors can be misleading, as the brightness between FITC and PE are quite different (on a scale of 1-5, 5 being the brightness, PE rates a 5 and FITC rates a 3). If you are looking at products between companies, you should compare the same fluors to get an accurate comparison.
In addition to differences in fluorophore brightness, there can also be differences in optimal usage amount, which means that the concentrations between products may also be different, potentially contributing to less than optimal results. Furthermore, tandem dyes can potentially have distinctly different properties among different antibody manufacturers, in terms of brightness and signal-to-noise, so be aware when switching your source of tandem antibodies.
BioLegend Fluorophore Brightness Index
Clones
In addition to fluor differences, you should determine whether the clones in the comparisons you are making are the same. Different clones recognize different epitopes and have varying binding affinities. Therefore, a direct comparison between two different clones may not be accurate.
Lot Changes
If your results have changed over time, are you still using the same lot? If you have recently changed to a new lot, you may want to check its concentration to ensure you are still using the same amount of antibody. In addition, some test size lots have changed from a recommended usage of 20 µL/test to 5 µL/test. You may also want to compare the two lots side by side to confirm the new trend is lot-dependent.
Concentration Lookup Tool
Human Variation
Are you testing on human patients/samples? While mice can be genetically identical, humans, of course, present a lot more variation from person to person. You may need to increase your sample size to draw a conclusion for your data. In addition, the disease state/health, age, and ethnicity can all affect the expression levels of certain markers. Different batches of cell lines can also produce inconsistent results. Side-by-side comparisons should always be made with the same sample/cell types.

 Login / Register
Login / Register 











Follow Us