Growth Factors Can Cooperate to Promote Tumorigenesis
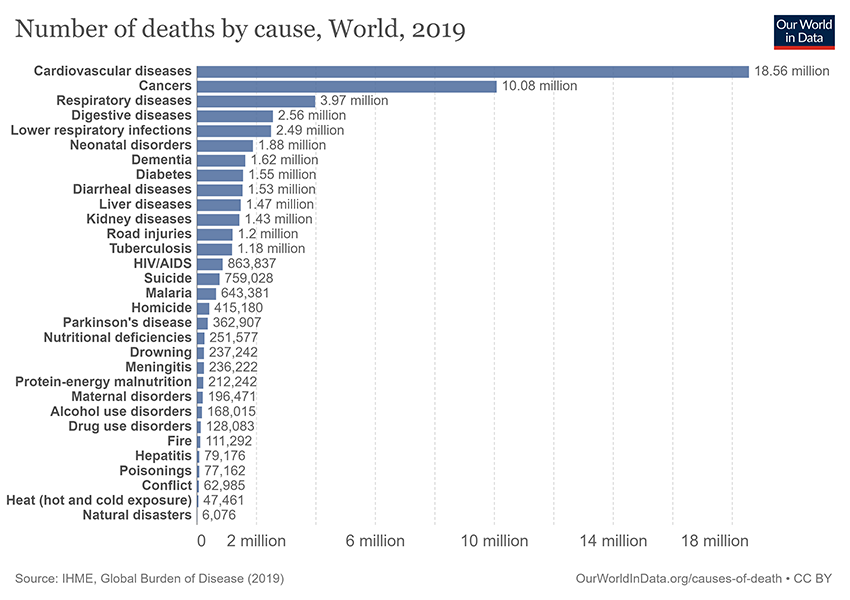
Cancer continues to rank as one of the largest health problems worldwide, accounting for one in every six deaths1 (Figure 1). Cancer progresses in a multistep process in which genetic alterations drive the transformation of healthy human cells into malignant derivatives2. The tumor microenvironment (TME) is complex and dynamic, comprising the cellular environment that harbors cancer stem cells, extracellular matrix (ECM), and a number of non-transformed cellular components, including stromal cells (normal connective tissue cells), fibroblasts, blood vessels, and immune cells2. Molecular and cellular crosstalk between malignant and nonmalignant cells creates a TME that affects cancer development and progression. In the context of cancer and the TME, a number of studies have focused predominantly on adaptive immune cells – T cells in particular given their cytotoxic capabilities2. It is not surprising then that there are current cancer treatments that target the TME and T cells; prime examples include immune checkpoint blockade and chimeric antigen receptor (CAR) T cell therapies.
The pathology of cancer involves aggressive changes at the cellular level, leading to aberrant signals between the living cells. Signaling pathways such as TGF-β (Transforming growth factor-β), Wnt (Wingless/Integrated), and EGF (epidermal growth factor) evolved to regulate growth and development in mammals. However, these growth factors are also implicated for tumorigenesis if failure or aberrant expression of components in these pathways occurs.
To better understand the TME, we need to closely investigate the signaling pathways that help form and maintain it. TGF-β is a pleiotropic molecule, member of a superfamily that includes activin and bone morphogenetic proteins (BMPs), exerting a broad range of functions. The TGF-β superfamily is critical in signaling in embryogenesis and, in adults, safeguards against cancer through inhibitory effects on the cell cycle and proliferation of normal epithelial cells. Paradoxically, TGF-β induces tumor progression and metastasis in advanced cancers as a result of tumor cells accumulating mutations in components of the TGF-β signaling pathway. This allows tumor cells to escape the growth inhibitory effects of TGF-β3.
Wnt signaling pathways also regulate many aspects of mammalian development and play important roles in determining cell fate, stem cell renewal, and maintaining stem and early progenitor cells. Similar to TGF-β, abnormalities in Wnt signaling play crucial roles in cancer stem cell renewal, cell proliferation, and differentiation, ultimately driving tumor development and progression4. Cross-talk between TGF-β/BMP, Wnt, Hedgehog, and other pathways are critical to drive processes— cell proliferation, cell migration, and angiogenesis— that underlie embryonic development and adult tissue homeostasis, but aberrant signaling in any of these pathways leads to pathologies in which those same processes are hallmarks5.
At BioLegend, we offer an array of tools to support your research on these signaling pathways and their contribution to the TME. We have an extensive offering of recombinant cytokines and growth factors, each of which undergoes rigorous quality testing and verification of bioactivity using functional assays.
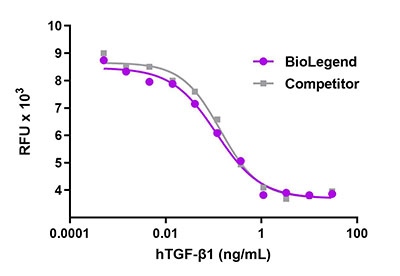
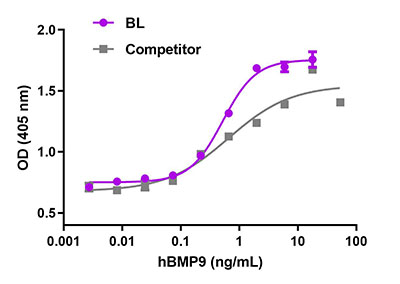 Recombinant human BMP-9 induces the production of alkaline phosphatase in the embryonal carcinoma-derived chondrogenic ATDC5 cell line in a dose dependent manner. BioLegend’s protein was compared side-by-side and it outperformed a leading competitor’s equivalent product.
Recombinant human BMP-9 induces the production of alkaline phosphatase in the embryonal carcinoma-derived chondrogenic ATDC5 cell line in a dose dependent manner. BioLegend’s protein was compared side-by-side and it outperformed a leading competitor’s equivalent product.
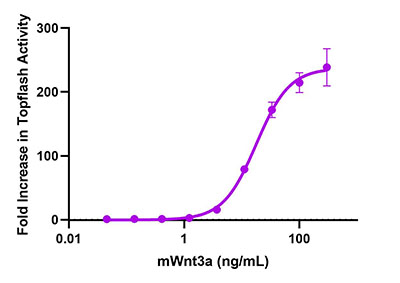
Also critical is the ability to accurately quantify the expression levels of growth factors in response to cancer progression and related cellular processes. Our LEGENDplex™ Human Growth Factor Panel is a flow-based multiplex assay that allows for the simultaneous quantification of 13 human growth factors, many of which play key roles in cancer development and progression.
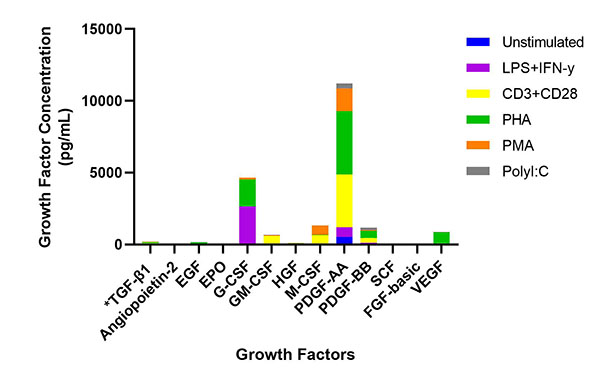
Human PBMCs (1 x 106 cells/mL) were cultured with either LPS (1 μg/mL) + IFN-γ (10 μg/mL), anti-CD3/CD28 (1 μg/mL), PHA (1 mg/mL), PMA (50 ng/mL) for 24 hours, Poly I:C (2.5 μg/mL), or Control (unstimulated PBMC). All groups (except PMA) were stimulated at 37°C for 72 hours. Supernatants were collected and assayed with the LEGENDplex™ Human Growth Factor Panel. *For this representative study, TGF-β1 was included as target analyte in place of TGF-α that is typically part of this panel.
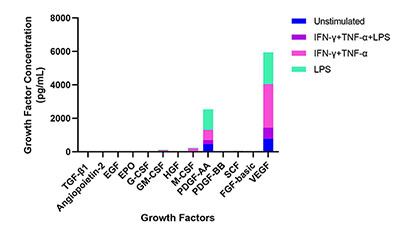
- IFN-γ (100 ng/mL) + TNF-α (100 ng/mL) + LPS (1 μg/mL)
- IFN-γ (100 ng/mL) + TNF-α (100 ng/mL)
- LPS (1 µg/mL)
In addition to our multiplex panels, our ELISA sets and kits can precisely quantify individual growth factors in tissues or cells of interest. For example, the granulocyte colony-stimulating factor (G-CSF) is often used to stimulate white blood cell production as treatment after chemotherapy, but G-CSF also exhibits tumor-promoting effects on tumor cells and the TME2. Learn more about our ELISA MAX™ Deluxe Set Mouse G-CSF and related ELISAs for CSF to advance your research on the multiple, complex roles of this growth factor.
In summary, the cellular and non-cellular components of the TME utilize signaling pathways to drive tumor emergence and development. Our expertly-crafted recombinant growth factors and immunoassay tools help researchers gain better insights into the cellular and molecular mechanisms underlying the TME. It is our hope that such insights lead to novel ways to disrupt cancer development or develop safe and effective therapeutic strategies to combat cancer. Learn more how our recombinant proteins and multiplex assays can help your research combat cancer. You can also download our handy infographic, and learn more about growth factors as disease biomarkers and therapeutics.
References
- Roser, Max. "Cancer." Our World in Data, 3 July 2015, ourworldindata.org/cancer.
- Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019 Sep 15;79(18):4557-4566. doi: 10.1158/0008-5472.CAN-18-3962. PubMed.
- Mallikarjuna, Pramod et al. “The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis.” Biomolecules vol. 12,5 635. 25 Apr. 2022, doi:10.3390/biom12050635. PubMed.
- Koni, Malvina et al. “The Wnt Signalling Pathway: A Tailored Target in Cancer.” International journal of molecular sciences vol. 21,20 7697. 18 Oct. 2020, doi:10.3390/ijms21207697. PubMed.
- Luo, Kunxin. “Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways.” Cold Spring Harbor perspectives in biology vol. 9,1 a022137. 3 Jan. 2017, doi:10.1101/cshperspect.a022137. PubMed.
 Login / Register
Login / Register 






Follow Us