| Welcome to the BioLegend Interleukin Receptors page, where you can find information on Interleukin cytokine receptors including ligands, receptor pairings, diagrams, and research products. This page only covers receptors for Interleukins, not chemokines, interferons, or other growth factors. Browse by interleukin family below to learn more. | Analyze multiple cytokines simultaneously with LEGENDplex™ |
Interleukin Receptors
| Members of the immunoglobulin superfamily receptors (IgSF) are transmembrane proteins defined by their structural similarity to immunoglobulins. They often contain an amino-terminal extracellular domain that holds the characteristic immunoglobulin fold. IgSF receptors are typically involved with cell adhesion and the interaction between T cells and antigen presenting cells. |
| IL-1 | IL-18 | IL-33 | IL-36 | IL-37 |
|
|
| As their name implies, the IL-17 Receptors bind the IL-17 family of cytokines (IL-17A, A/F, B, C, D, E, and F). This family of receptors consists of 5 members (IL-17RA, RB, RC, RD, and RE). There is no particular structural similarity between the IL-17 receptors and any other family of receptors. The function of these receptors varies with their ligands. IL-17A and F are known for their inflammatory properties, while IL-17E (IL-25) promotes a Th2 response. |
| IL-17A | IL-17B | IL-17C Set 1 | IL-17C Set 2 |
| IL-17D | IL-17E | IL-17A/IL-17F/IL-17A/F | IL-17RD |
|
|
| Type I cytokine receptors are transmembrane receptors that recognize cytokines with four α-helical strands. The receptors of this family share a common amino acid motif (WSXWS) in the extracellular portion of the cell membrane. This family can be further subdivided based on whether the receptor complex utilizes the gp130, common beta chain (IL-2Rβ), or common gamma chain (IL-2Rγ) subunits. The functions of these family members are diverse, playing a major role in the development and function of immune and blood cells. |
| IL-2 | IL-3 | IL-4 | IL-5 | IL-6 | IL-7 | IL-9 | ||||||
|
||||||||||||
| IL-21 | IL-23 | IL-27 | IL-31 | IL-32 | IL-34 | IL-35 | ||||||
|
|
| Type II cytokine (T2) receptors are similar in structure to type I cytokine receptors. However, they differ in the placement of a conserved cysteine sequence and T2 receptors do not possess the WSXWS amino acid motif. T2 receptors are known for mediating the function of Type I and II interferons and the interleukin-10 family. The functions of these receptors vary greatly. For example, interferon-γ is known for its potent inflammatory effects, while IL-10 is an immunosuppressive cytokine. |
| IL-10 | IL-19/IL-20/IL-24 | IL-22 Set 1 | IL-22 Set 2 | |||
|
||||||
|
|
| IL-1α, IL-1β, and IL-1RA are all capable of binding to the IL-1R1 and IL-1R2 receptors. This signaling complex also requires the presence of IL-1R Accessory Protein (IL-1RAcP). While IL-1α and IL-1β are mediators of inflammation, IL-1RA does not have agonistic effects or initiate downstream signaling. Instead, IL-1RA acts as competitive inhibitor of IL-1 receptor binding. |
| IL-1α | IL-1β | IL-1 RA | IL-1R1 | IL-1R2 | IL-1RAcP |
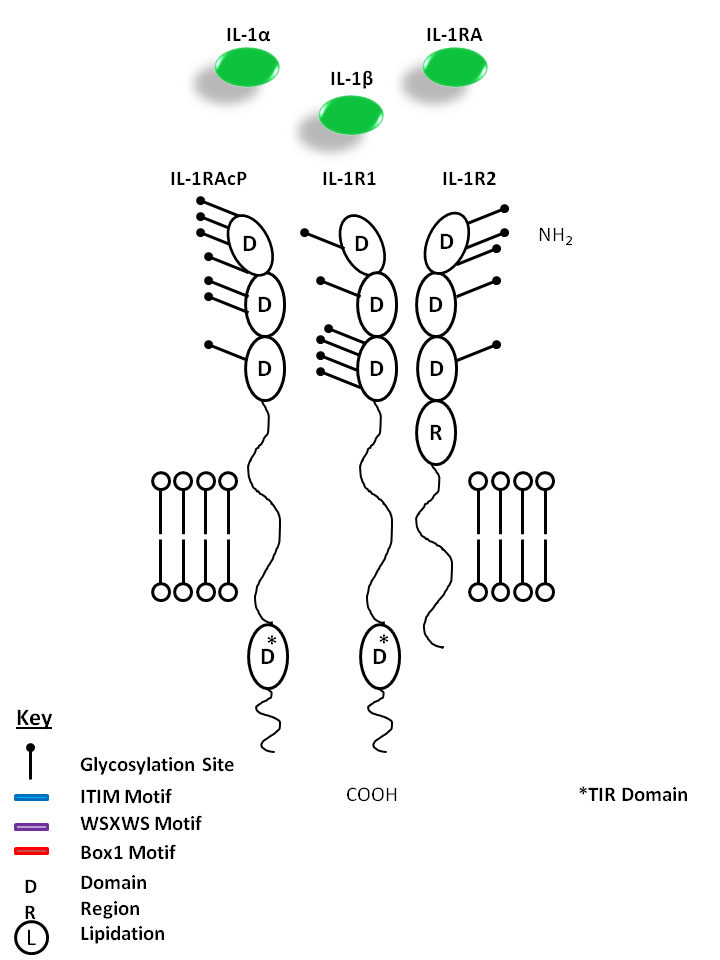 |
| IL-18 is bound by IL-18Rα and IL-18R Accessory Protein (IL-18RAcP). A proinflammatory cytokine, IL-18 is known to induce IFN-γ, IL-2, and GM-CSF production from Th1 cells. |
| IL-18 | IL-18RAcP | IL-18Rα |
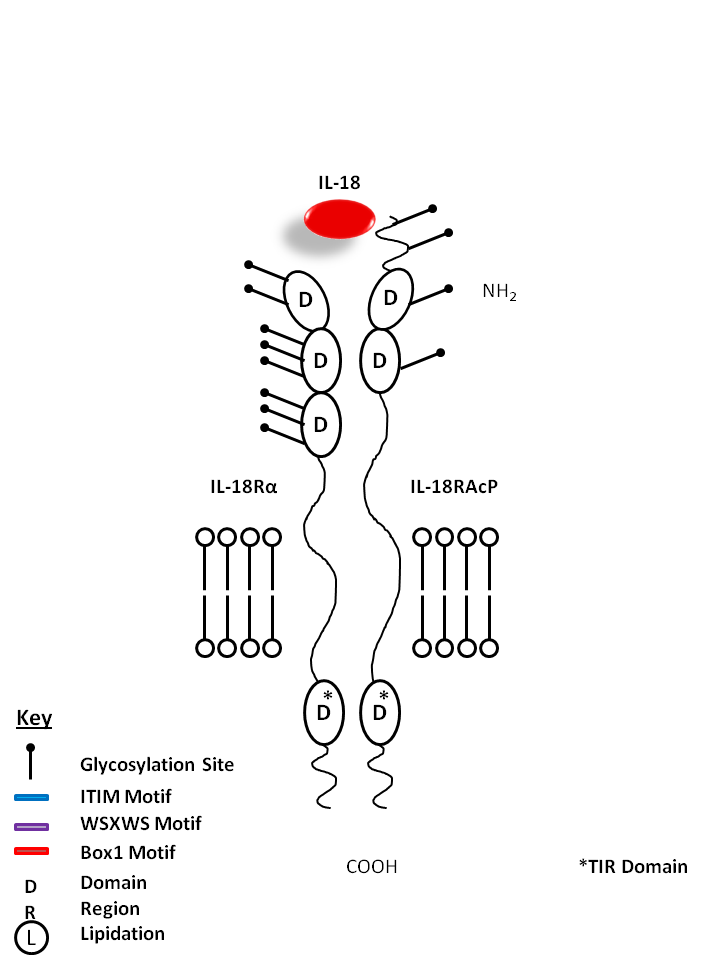 |
| IL-33 is bound by IL-1R Accessory Protein (IL-1RAcP) and IL-1 Receptor-Like 1 (IL-1RL1). Upon in vivo injection, IL-33 has been known to induce production of IL-5, IL-13, IgE, and IgA. IL-33 is known to be a chemoattractant for Th2 cells and stimulates them to produce Th2-associated cytokines. |
| IL-33 | IL-1RL1 | IL-1RAcP |
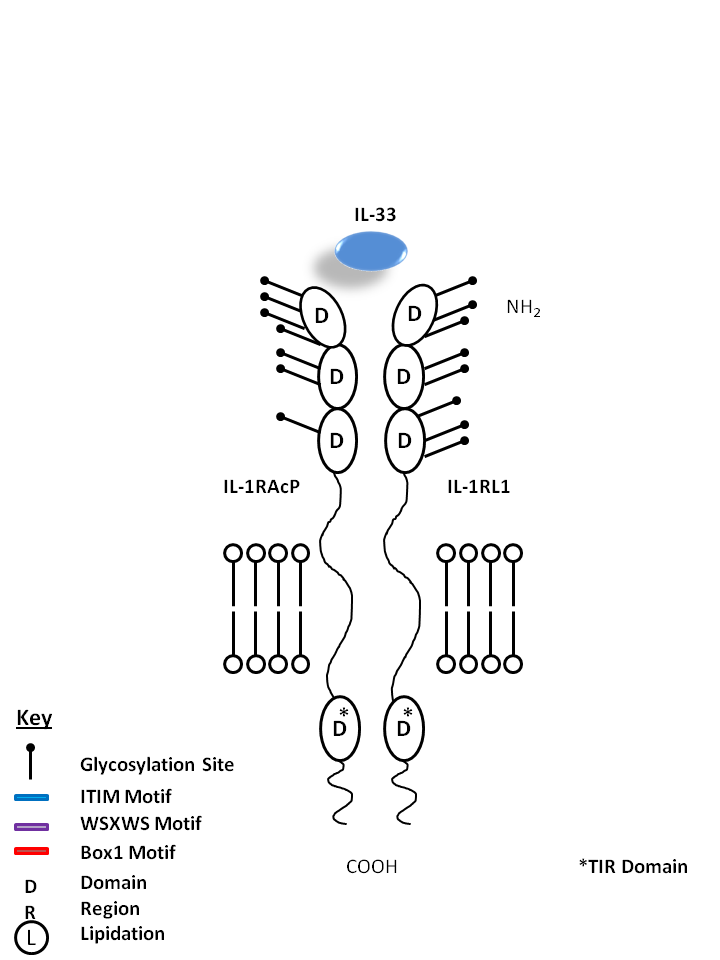 |
| IL-36α, IL-36β, IL-36γ, and IL-36RA are all bound by IL-1R Accessory Protein (IL-1RAcP) and IL-1 Receptor Like 2 (IL-1RL2). IL-36α, β, and γ are known to promote proinflammatory cytokine/chemokine production (CXCL8). IL-36RA, on the other hand, is believed to hold antagonistic function, acting as a competitive inhibitor. |
| IL-36α | IL-36β | IL-36γ | IL-36RA | IL-1RL2 | IL-1RAcP |
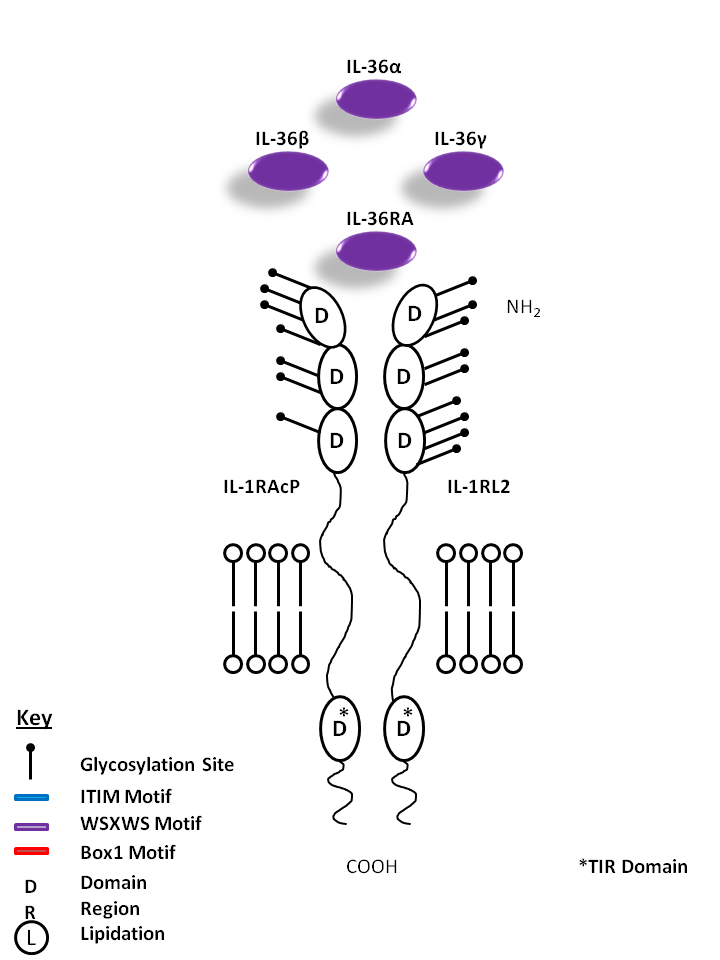 |
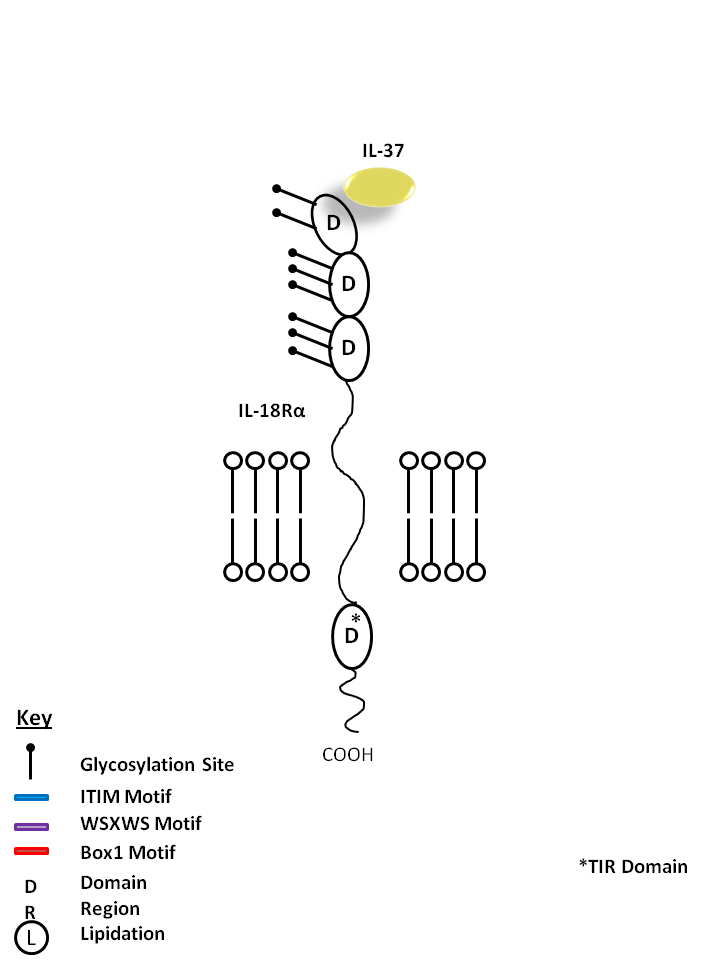
| IL-37 is bound by IL-18Rα. IL-37 is known to curb excessive innate inflammatory responses, limiting the action of IL-1α, IL-1β, IL-6, CCL12, CSF1, CSF2, CXCL13, and IL-23A. It is also known to inhibit dendritic cell activation |
| IL-37 | IL-18Rα |
 |
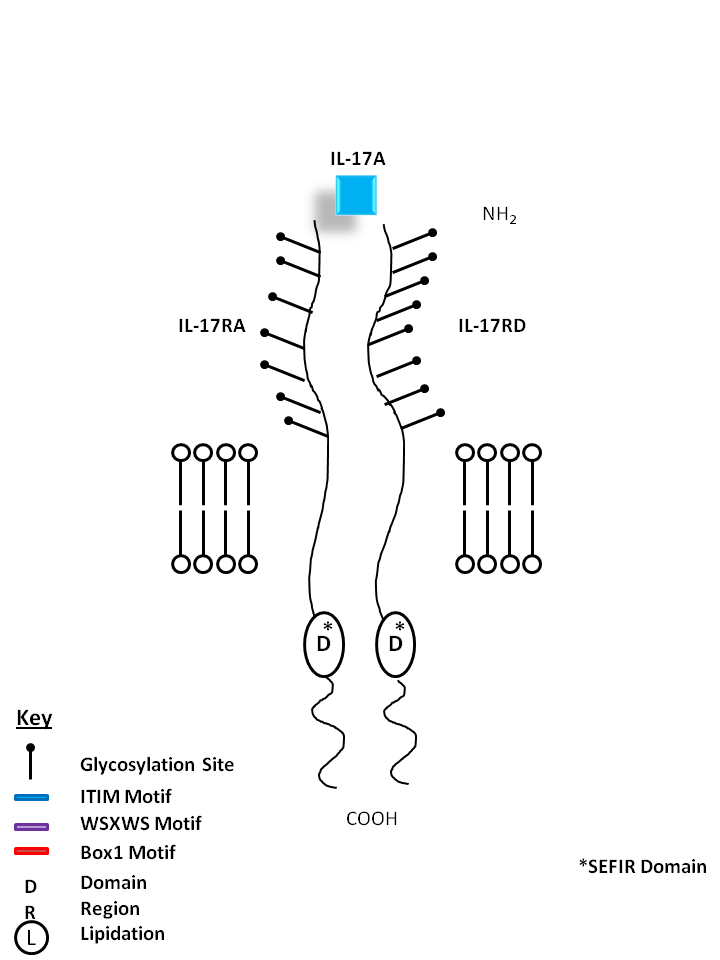
| IL-17A can be bound by IL-17RA and IL-17RD. IL-17A is made primarily by Th17 cells. It can induce chemokine and cytokine (e.g. IL-6, IL-8) from fibroblasts, chemoattract neutrophils, and is essential for several autoimmune diseases. |
| IL-17A | IL-17RA | IL-17RD |
 |
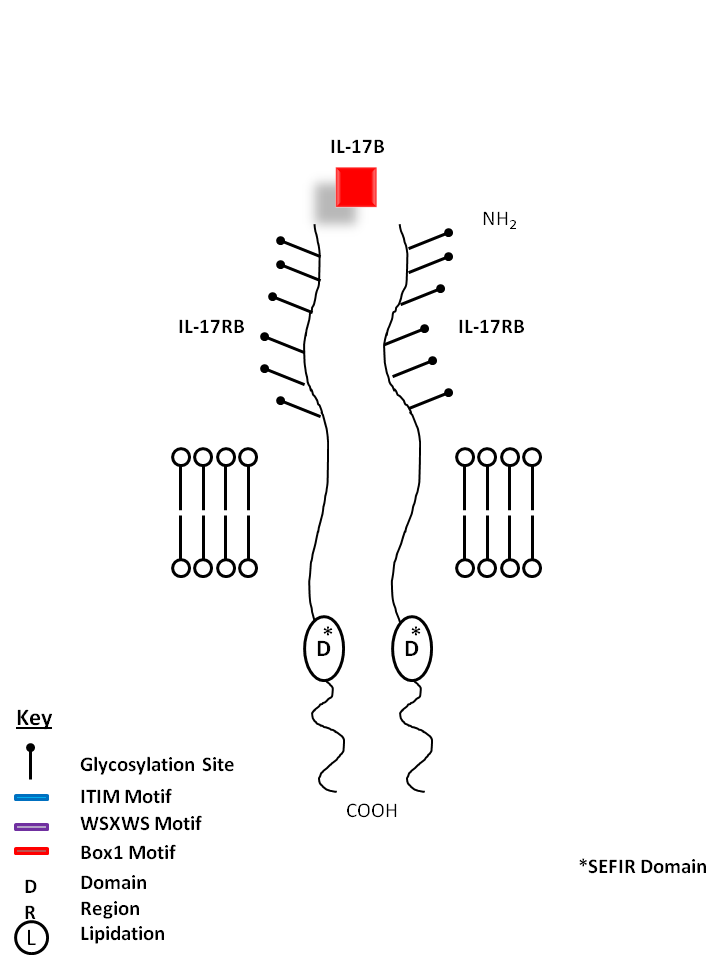
| IL-17B is bound by a homodimer of IL-17RB. IL-17B shares the most homology with IL-17D. IL-17B expression is typically restricted to neuronal cells. It is reported to stimulate the release of TNF-α and IL-1β from a monocytic cell line. |
| IL-17B | IL-17RB |
 |
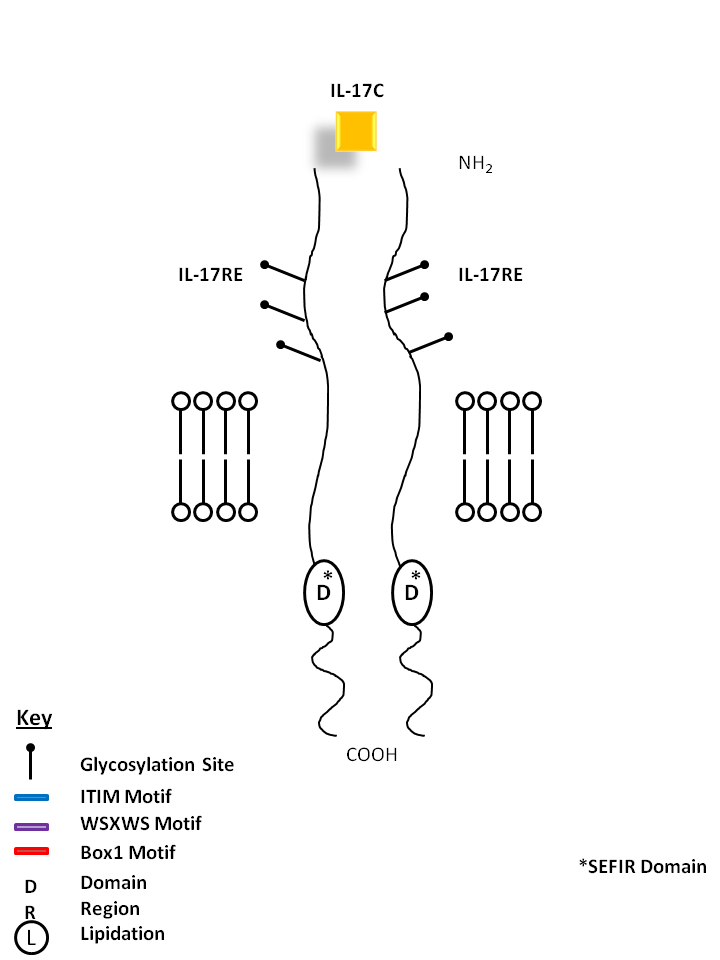
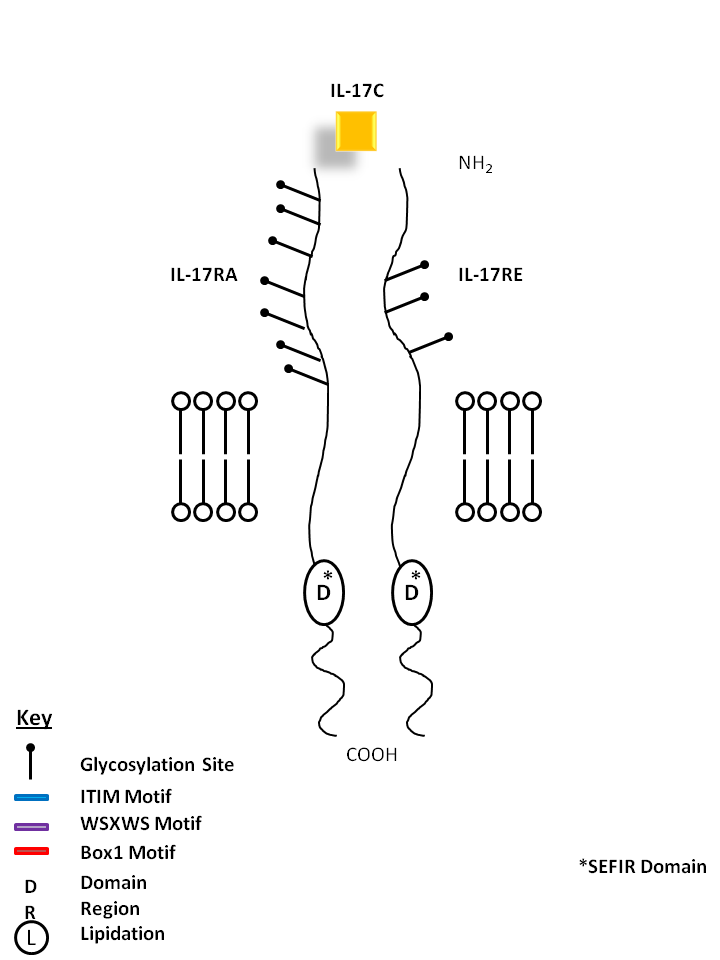
| IL-17C is bound by a homodimer of IL-17RE. Similar to IL-17B, it can stimulate TNF-α and IL-1β from a monocytic cell line. Its expression is typically restricted to activated T cells. |
| IL-17C | IL-17RE |
 |
| IL-17C can be bound by a heterodimer of IL-17RA and IL-17RE. Similar to IL-17B, it can stimulate TNF-α and IL-1β from a monocytic cell line. Its expression is typically restricted to activated T cells. |
| IL-17C | IL-17RA | IL-17RE |
 |
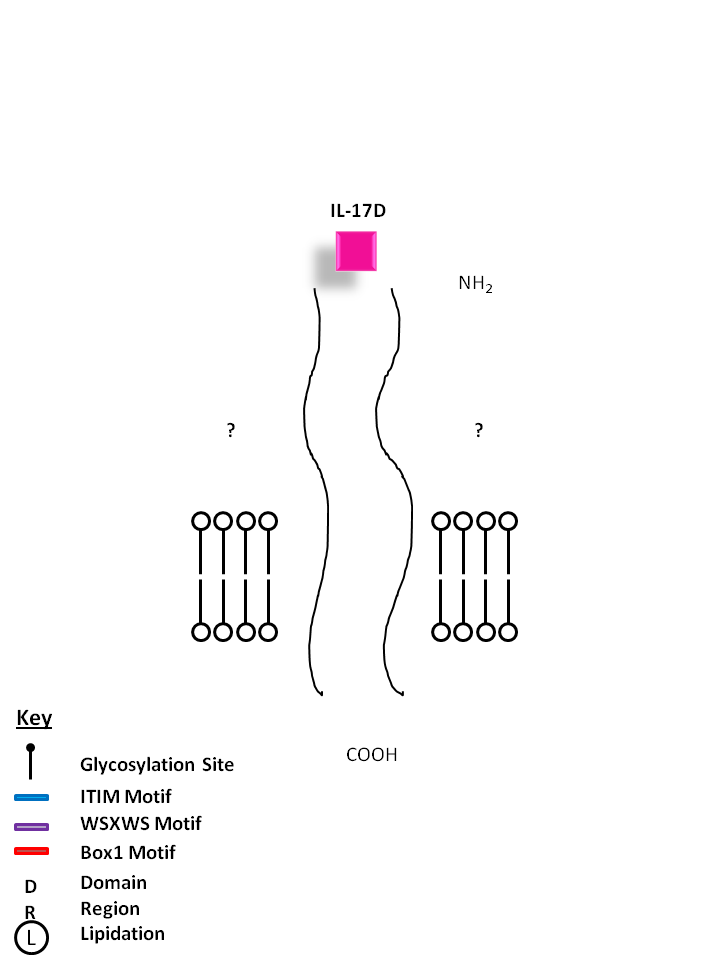
| The receptors for IL-17D are currently unknown. IL-17D shares the most homology with IL-17B. It exerts biological function by inducing the production of myeloid growth factors and chemokines like IL-6 and IL-8, and GM-CSF. |
 |
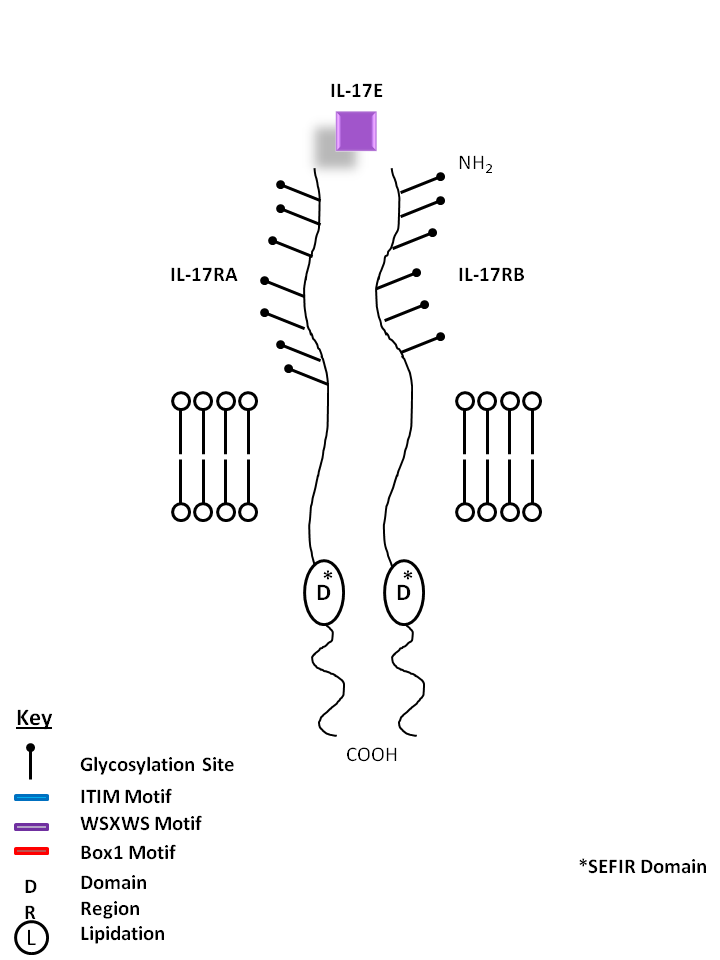
| IL-17E, also known as IL-25, is bound by IL-17RA and IL-17RB. IL-17E stimulates production of IL-8 and promotes Th2 phenotype development. |
| IL-17E | IL-17RA | IL-17RB |
 |
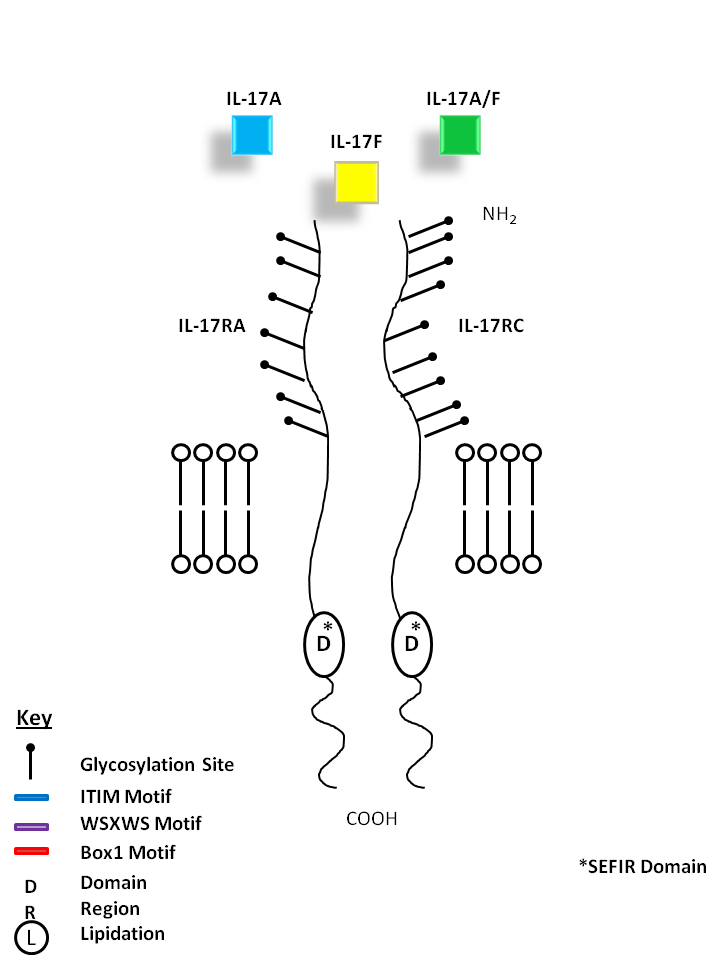
| IL-17A (homodimer), IL-17A/F (heterodimer), and IL-17F (homodimer) can be bound by IL-17RA and IL-17RC. IL-17A and IL-17F show the most homology among the IL-17 cytokine family. IL-17A and IL-17F share similar biological functions, recruiting granulocytes and inducing inflammation. While IL-17A is known to promote angiogenesis, IL-17F inhibits it. |
| IL-17A | IL-17A/F | IL-17F | IL-17RA | IL-17RC |
 |
| IL-17RD is known to form a homodimer receptor, but its ligand is currently unknown. |
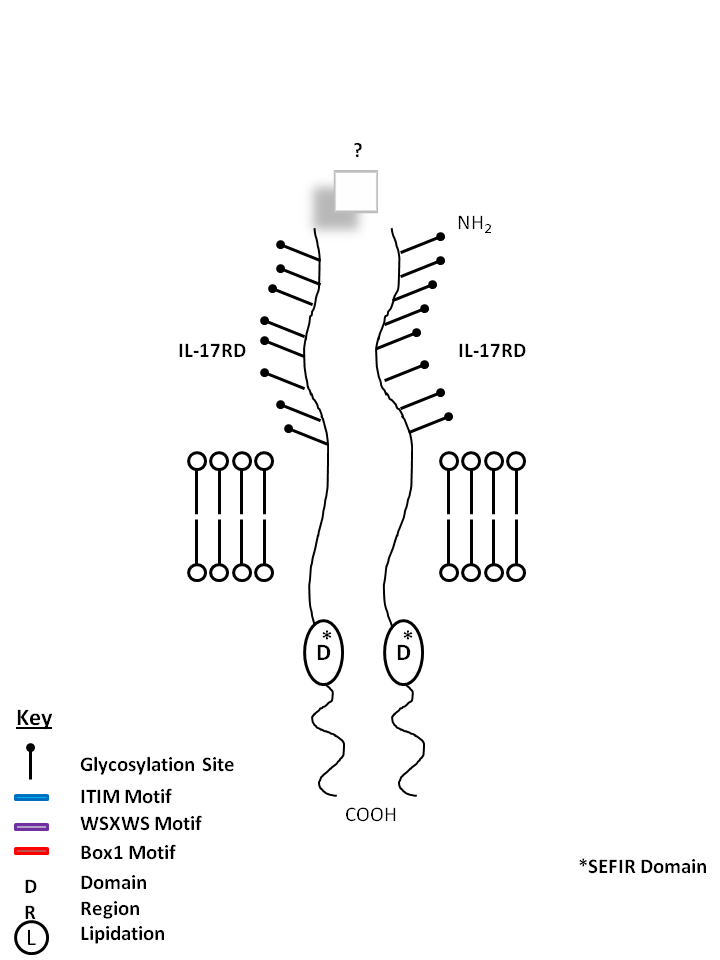 |
| The IL-2 receptor is composed of three parts: IL-2Rα, IL-2β, and IL-2γ. IL-2 is known to promote the proliferation of T lymphocytes and B cells. It is also required for the expansion and maintenance of Regulatory T cells. |
| IL-2 | IL-2Rα | IL-2Rβ | IL-2Rγ |
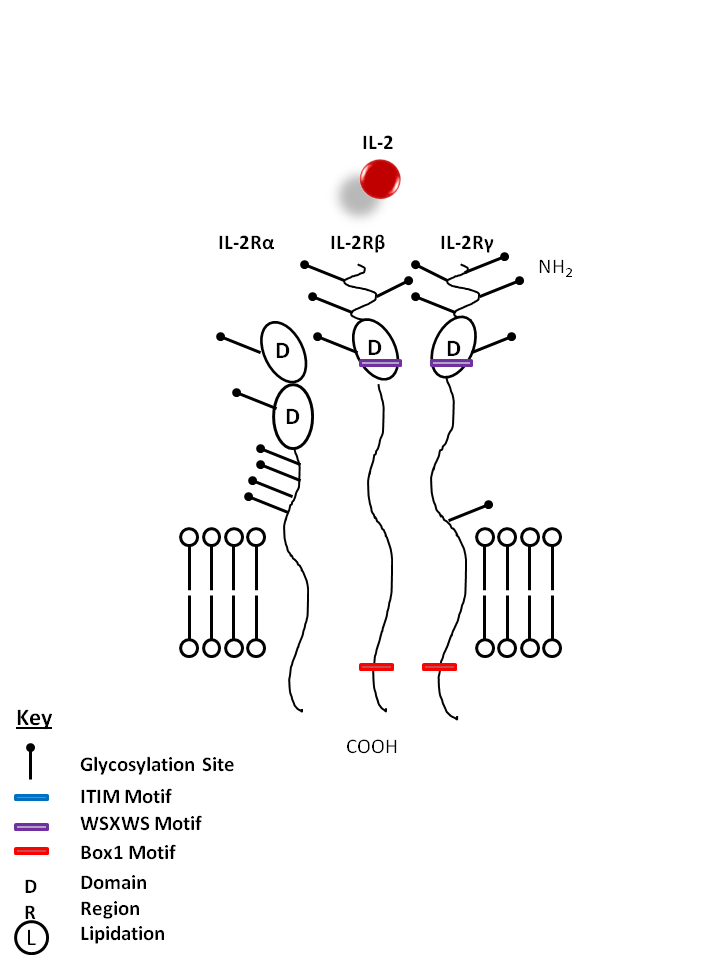 |
| IL-3 is bound by IL-3Rα and Colony Stimulating Factor 2 Receptor Beta (CSF2RB). IL-3 has several functions, but is particularly important for the differentiation of hematopoietic stem cells into granulocytes, macrophages, erythroid cells, megakaryocytes, and mast cells. |
| IL-3 | IL-3Rα | CSF2RB |
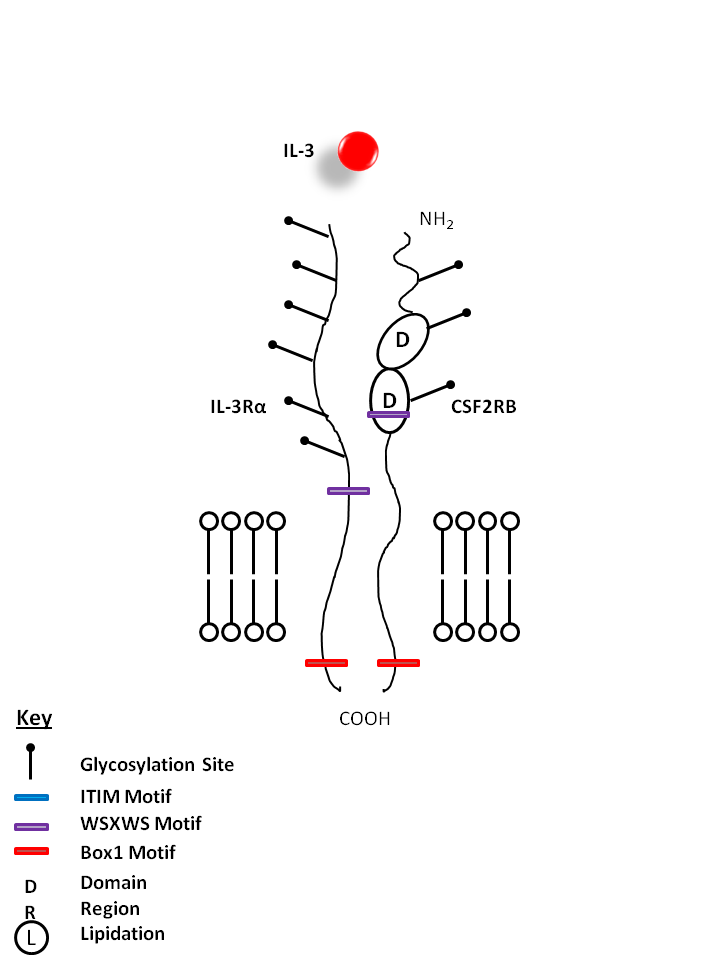 |
| IL-4 is capable of binding to two different complexes. The Type I complex consists of IL-4Rα and the common gamma chain (IL-2Rγ), while the type II complex consists of IL-4Rα and IL-13Rα1. IL-4 is primarily associated with creating a Th2 response, activating macrophages, and enhancing IgE mediated responses. |
| IL-4 | IL-4R | IL-2Rγ | IL-13Rα1 |
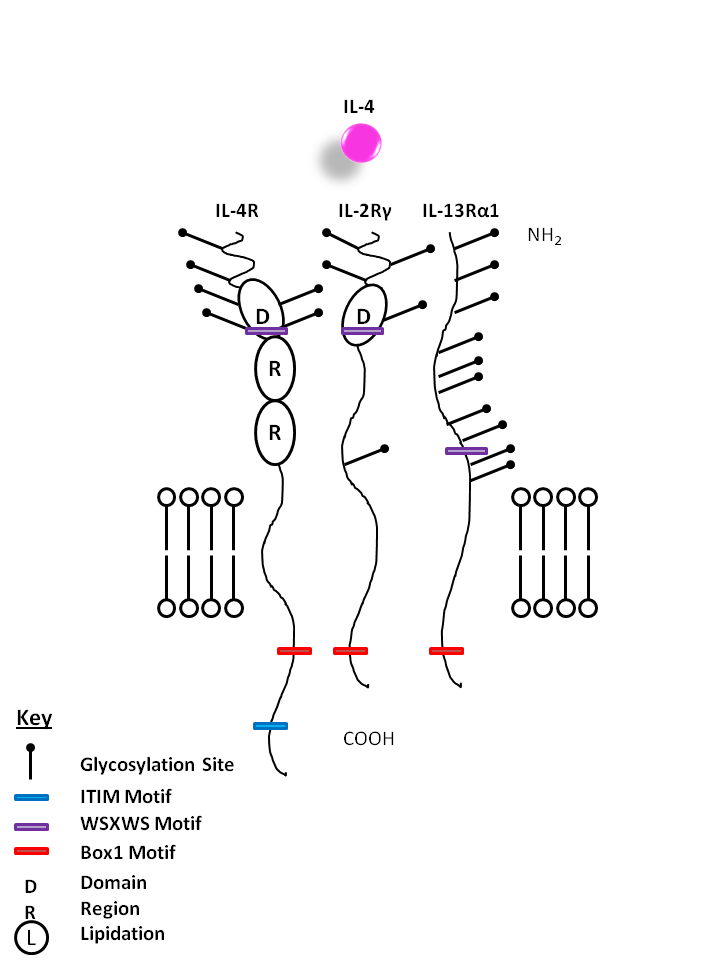 |
| IL-5 is bound by IL-5Rα and Colony Stimulating Factor 2 Receptor Beta (CSF2RB). IL-5 is known to aid in the maturation of BFU-E (burst forming unit erythroid) and activation of eosinophils. |
| IL-5 | IL-5Rα | CSF2RB |
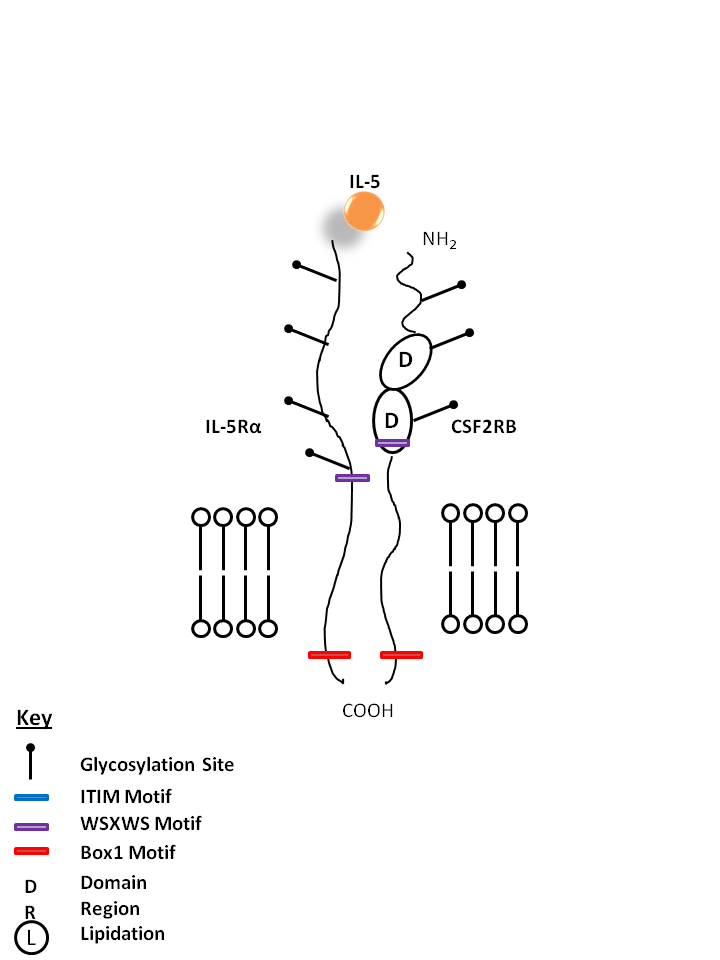 |
| IL-6 is bound by IL-6Rα and gp130. IL-6 has important functions in inflammation, acute phase reactions, and hematopoiesis. It is also known to be involved in Th17 cell differentiation. |
| IL-6 | IL-6Rα | gp130 |
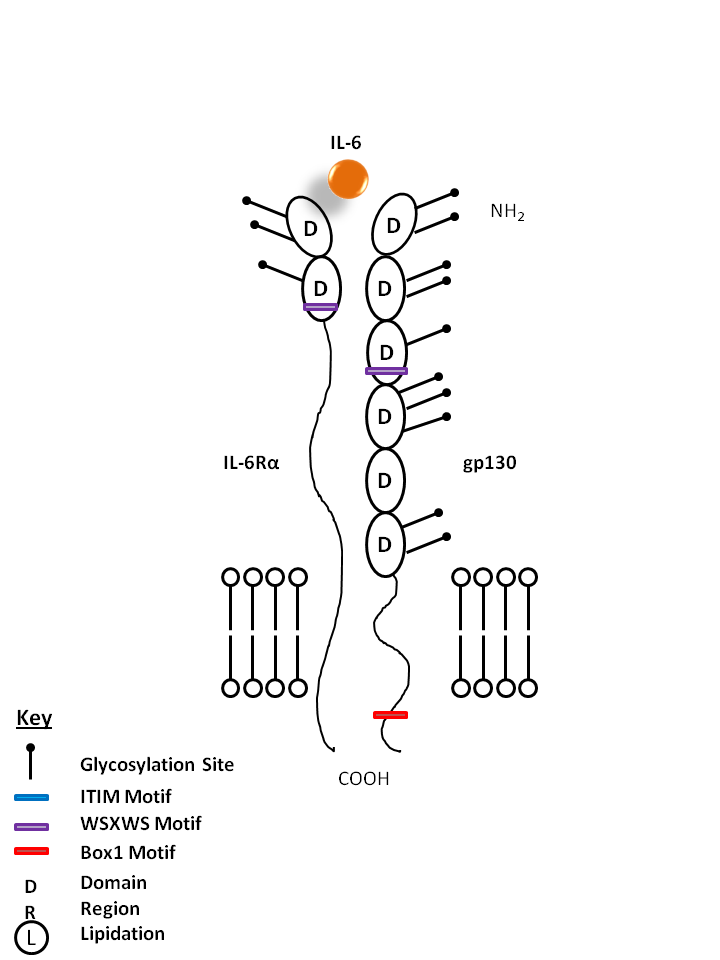 |
| IL-7 is bound by IL-7Rα and the common gamma chain (IL-2Rγ). IL-7 is vital for B cell maturation and T cell development. |
| IL-7 | IL-7Rα | IL-2Rγ |
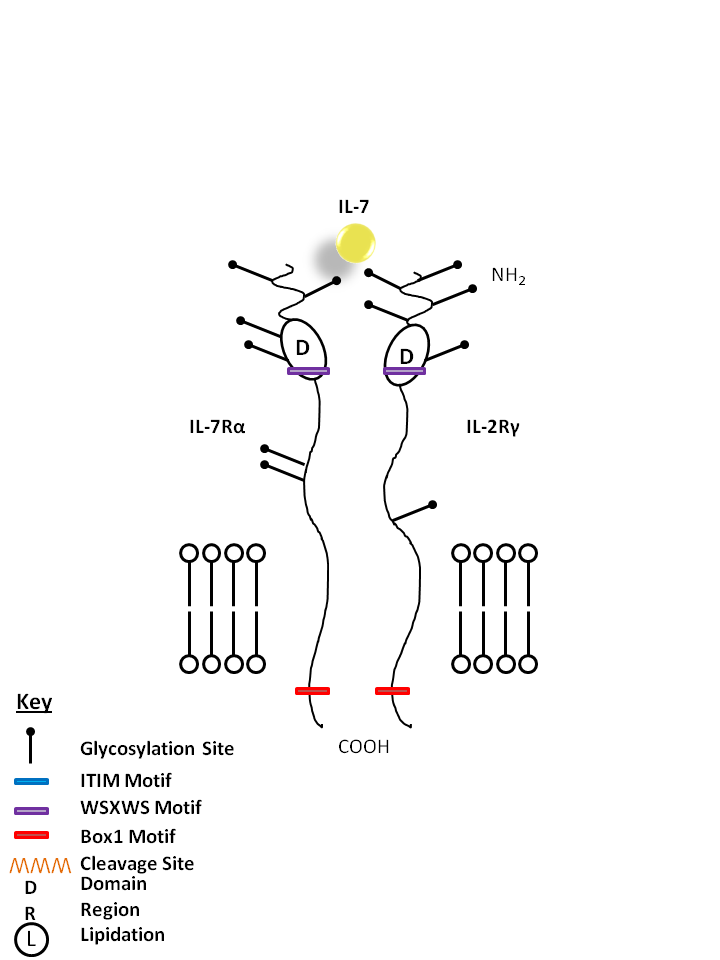 |
| IL-9 is bound by IL-9R and the common gamma chain (IL-2Rγ). IL-9 is known to enhance mast cell activity and it also promotes the growth of certain erythroid colonies and hematopoietic cell types. |
| IL-9 | IL-9R | IL-2Rγ |
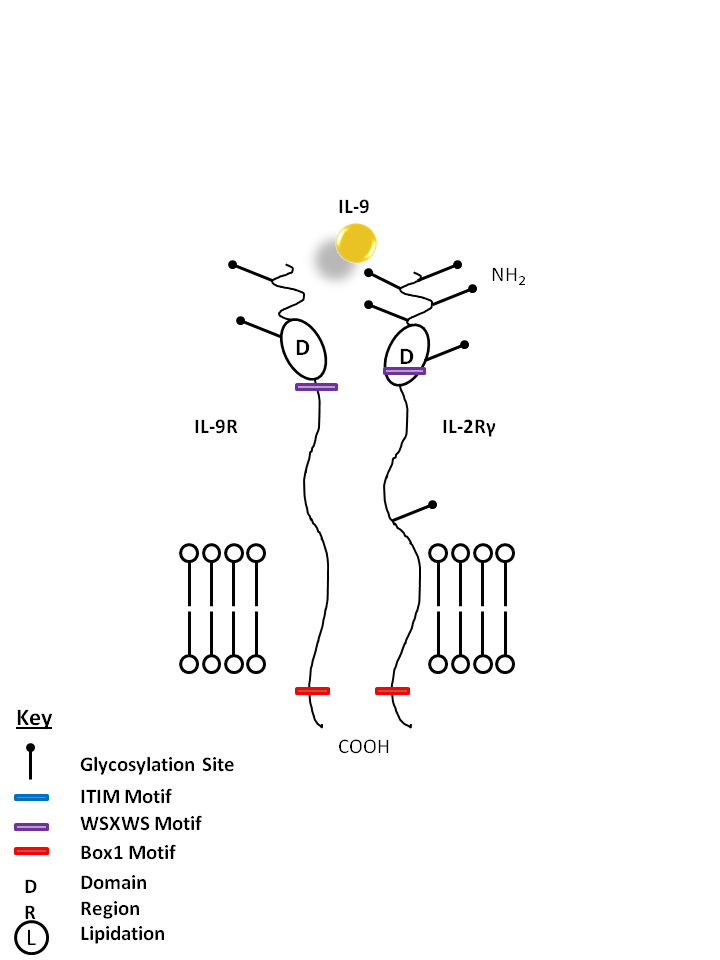 |
| IL-11 is bound by IL-11Rα and gp130. IL-11 is generally considered to be anti-inflammatory and is known to promote hematopoiesis, thrombropoiesis, Th2 polarization, and regulate macrophage differentiation. IL-11 often synergizes with other cytokines to produce effects similar to that of IL-6. |
| IL-11 | IL-11Rα | gp130 |
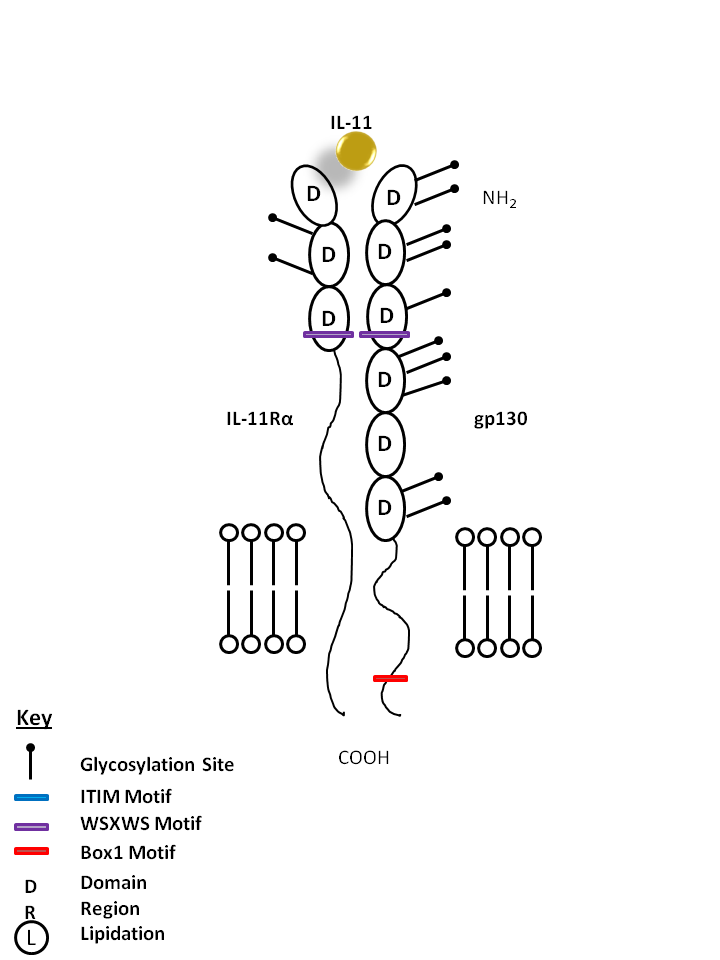 |
| IL-12 is bound by IL-12Rβ1 and IL-12Rβ2. IL-12 stimulates IFN-γ and TNF production from T and NK cells, driving Th1 differentiation. It helps act as a bridge between innate and adaptive immune responses. |
| IL-12 | IL-12Rβ1 | IL-12Rβ2 |
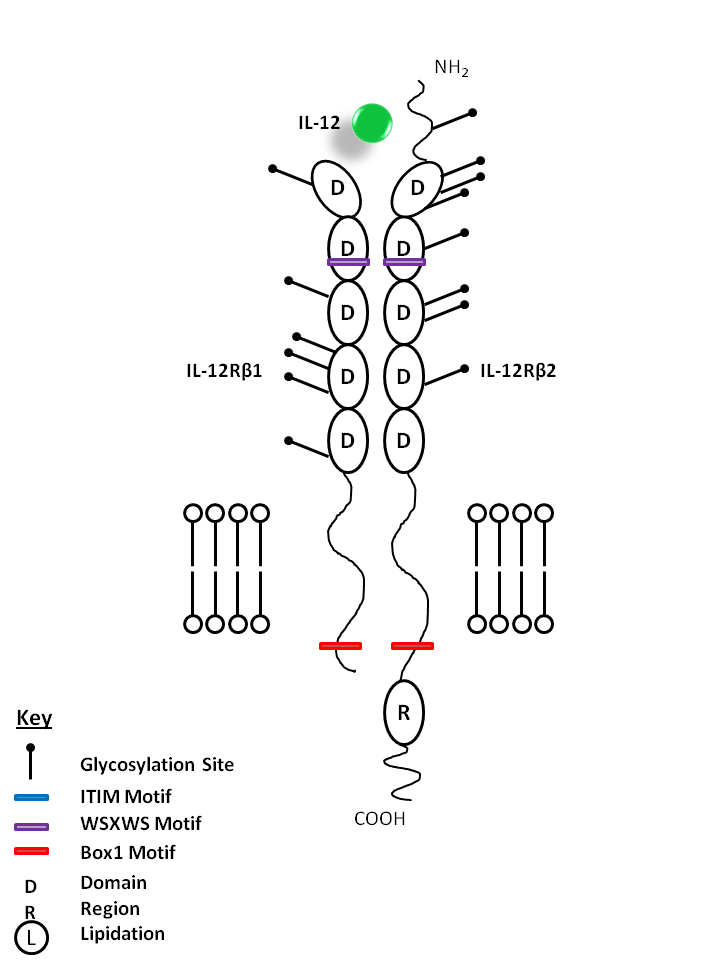 |
| IL-13 is bound by IL-4R and IL-13Rα1. IL-13 can also be bound by the IL-13Rα2 receptor on its own. IL-13 modulates monocytes/macrophages and B cells. It is also known to directly inhibit certain inflammatory cytokines like TNF-α and IL-1β. |
| IL-13 | IL-4R | IL-13Rα1 |
 |
| IL-13 can also be bound by the IL-13Rα2 receptor on its own. This interaction helps to limit the function of IL-13, as it is not known to mediate signaling. IL-13 modulates monocytes/macrophages and B cells. It is also known to directly inhibit certain inflammatory cytokines like TNF-α and IL-1β. |
| IL-13 | IL-13Rα1 |
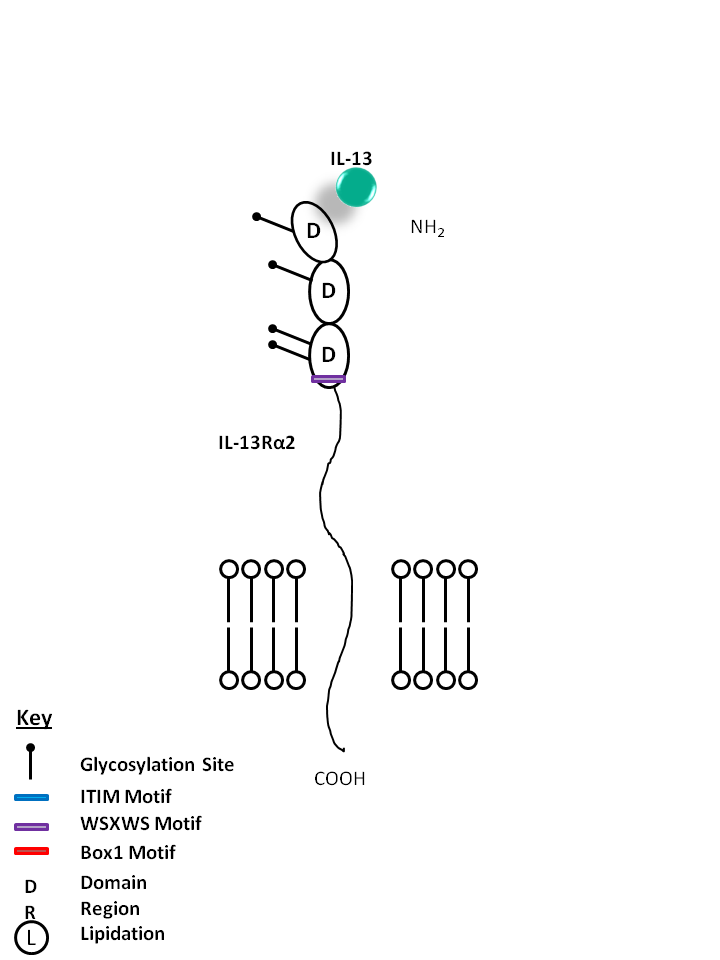 |
| IL-15 is bound by a receptor complex of IL-15Rα, IL-2Rβ, and IL-2Rγ. IL-15 promotes the proliferation of activated T, NK, and B cells. It is also effective in activating dendritic cells, neutrophils, and macrophages. |
| IL-15 | IL-12Rγ | IL-2Rβ | IL-15Rα |
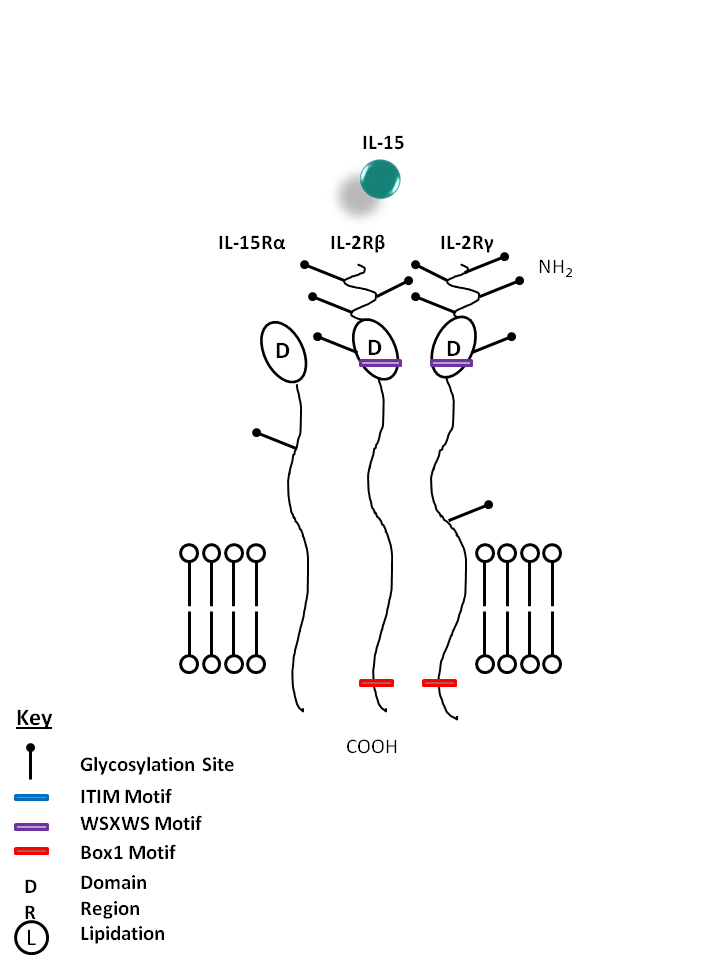 |
| IL-16 is bound by CD4 and CD9. IL-16 is a known chemoattractant for CD4+ T cells, eosinophils, and monocytes. It is also known to upregulate CD25 and MHC Class II expression. |
| IL-16 | CD4 | CD9 |
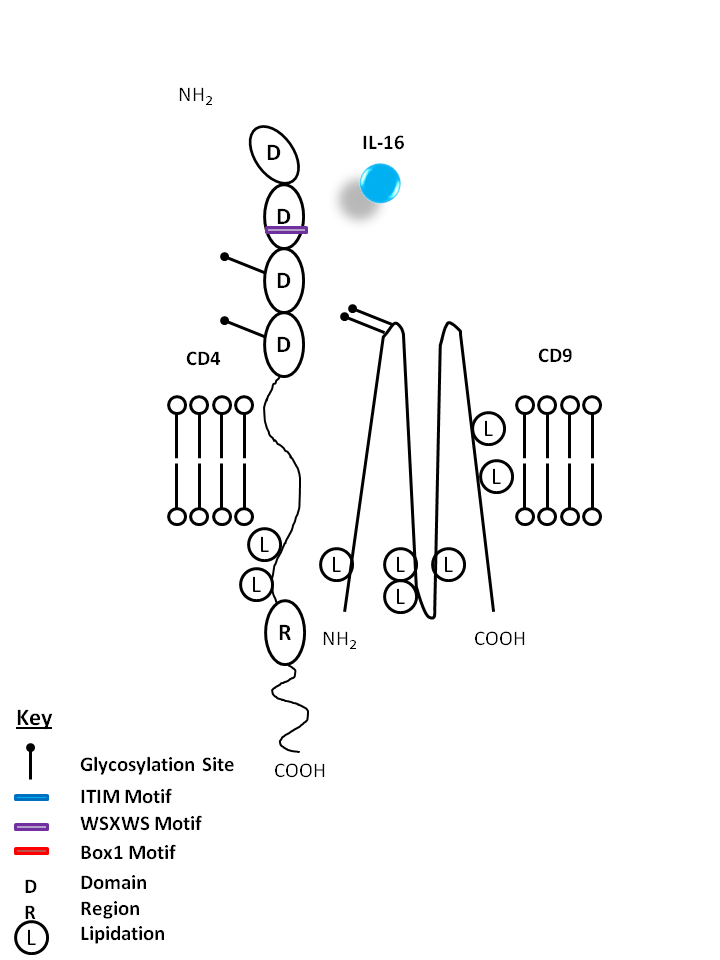 |
| IL-21 is bound by IL-21R and the common gamma chain (IL-2Rγ). IL-21 is one of the main products of Th17 cells, promoting proliferation of NK cells and cytotoxic T cells, development of B cells. IL-21 may also be implicated in several autoimmune diseases, as it has an autocrine function on Th17 cells. |
| IL-21 | IL-2Rγ | IL-21R |
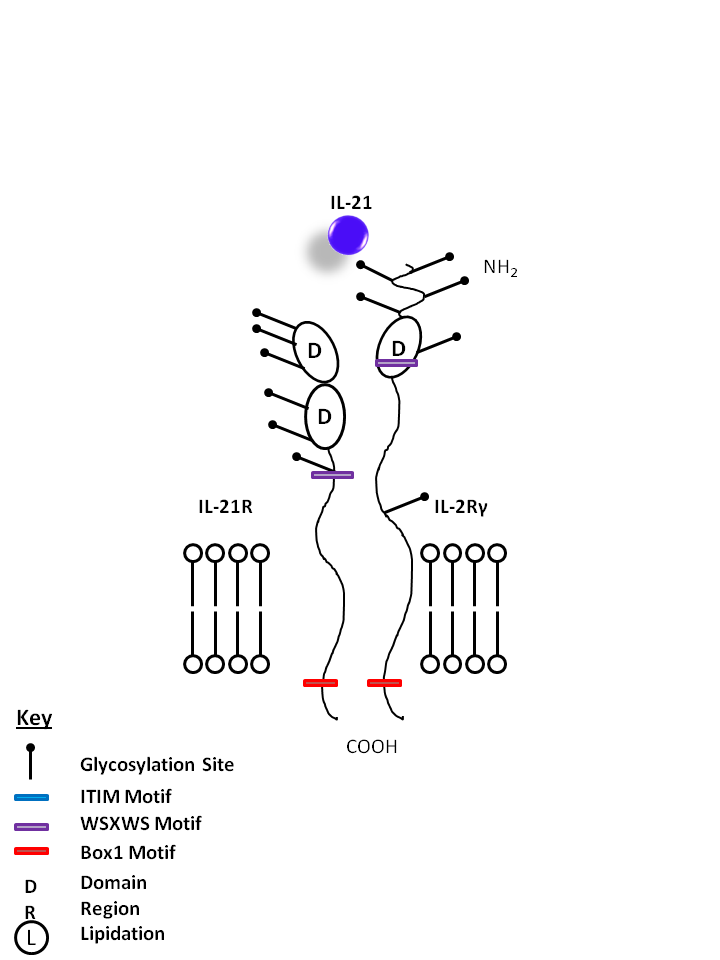 |
| IL-23 is bound by IL-12Rβ1 and IL-23R. IL-23 is vitally important for the maintenance and expansion of Th17 cells. As such, it induces the production of several Th17-related cytokines and factors, including IL-17A, IL-17F, IL-22, and RORγT. |
| IL-23 | IL-23R | IL-12Rβ1 |
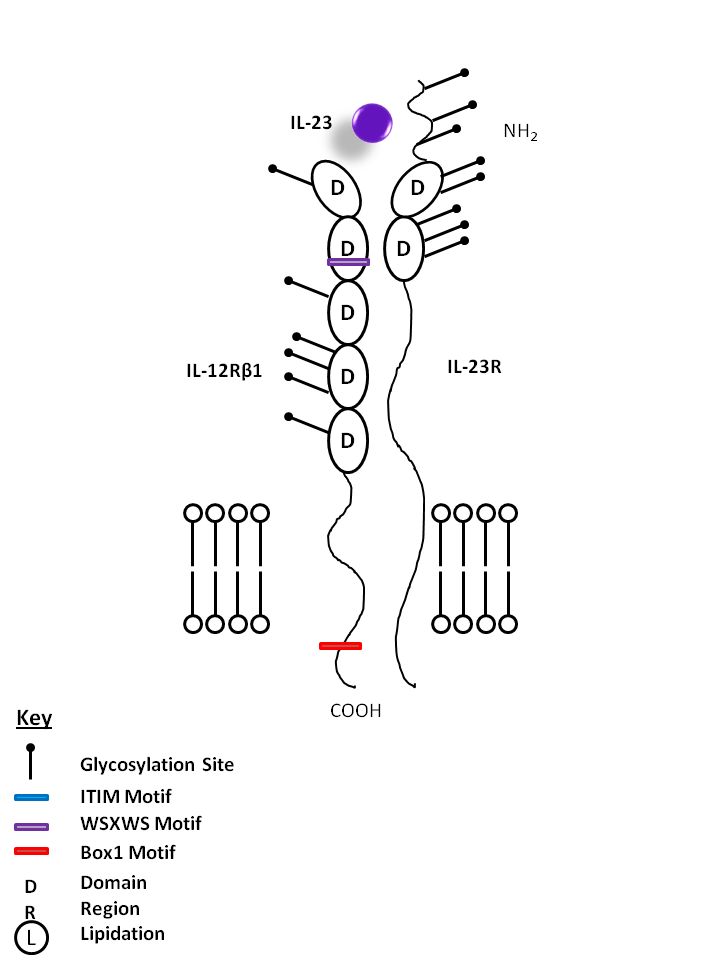 |
| IL-27 is bound by IL-27Rα and gp130. IL-27 is known to promote CD4+ T cell expansion and a Th1 phenotype. In vitro, it is also known to suppress cytokine production from CD4+ T cells and macrophages in an IL-10 dependent manner. |
| IL-27 | IL-27Rα | gp130 |
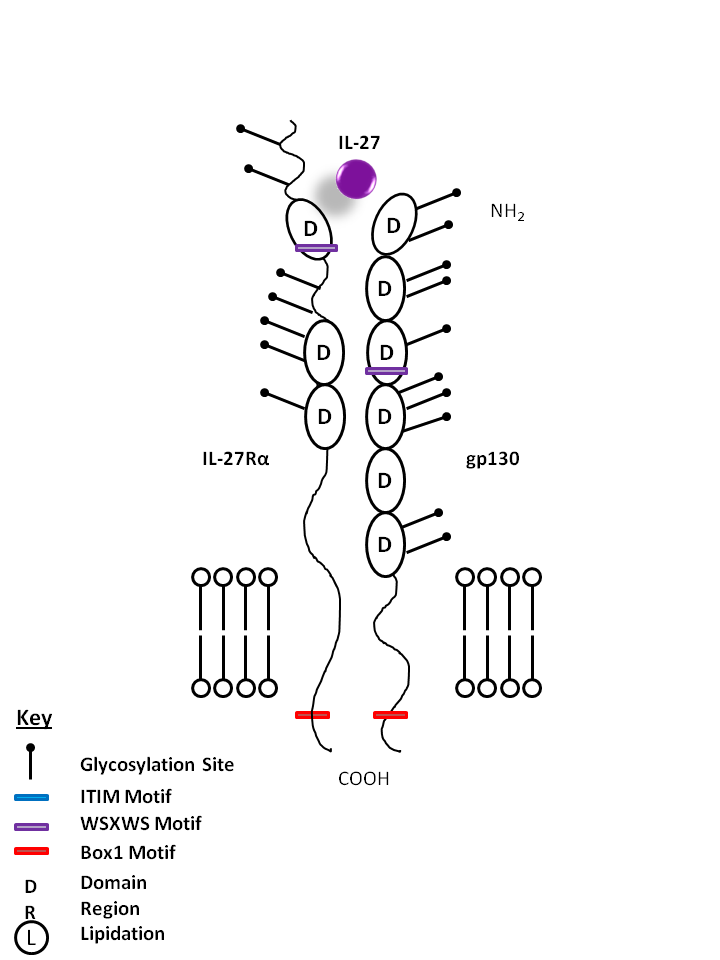 |
| IL-31 is bound Oncostatin M Receptor (OSMR) and IL-31Rα. IL-31 may be involved in skin immunity, contributing to itching in nonatopic dermatitis. |
| IL-31 | IL-31Rα | OSMR |
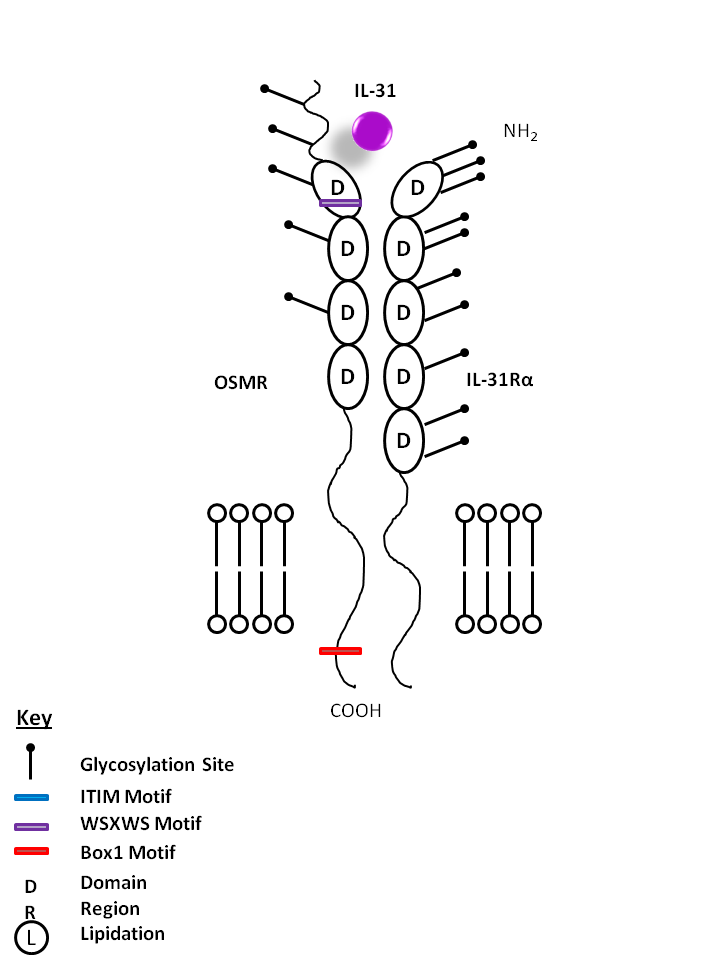 |
| The receptors for IL-32 are currently unknown. Consisting of four isoforms (α, β, γ, δ), IL-32 is known to induce proinflammatory cytokine expression in peripheral blood mononuclear cells. IL-32 has also been found to be highly expressed in several autoimmune diseases like Rheumatoid Arthritis and Inflammatory Bowel Disease. |
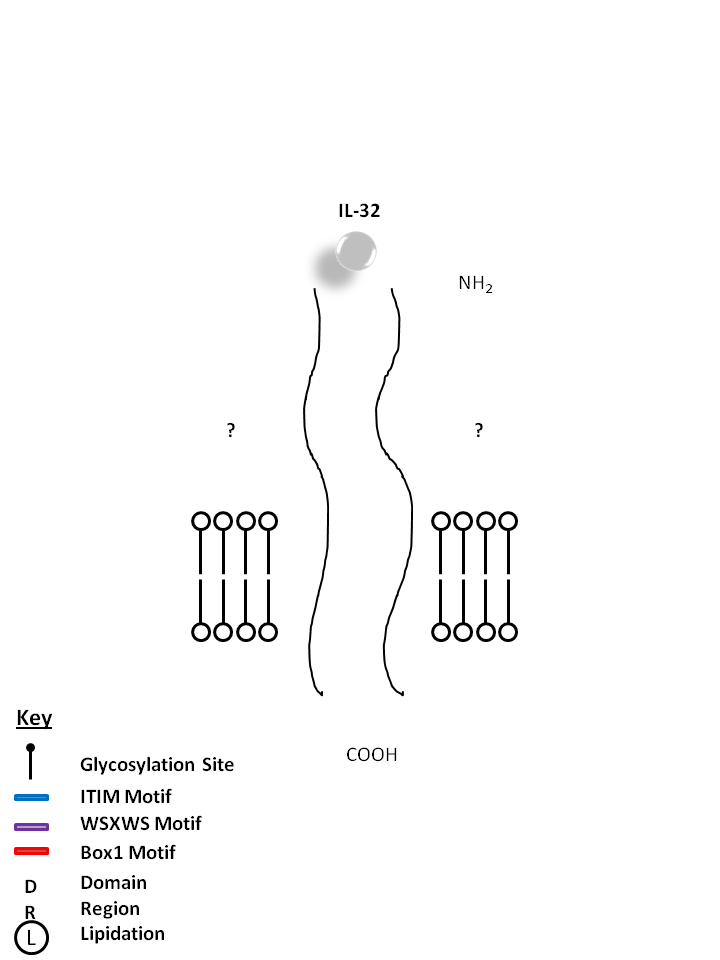 |
| IL-34 is bound by Colony Stimulating Factor 1 Receptor (CSF-1R). IL-34 acts primarily on monocytes, macrophages, and myeloid cells. It can increase monocyte viability, macrophage proliferation, and colony forming unit-macrophage formation in human bone marrow culture. |
| IL-34 | CSF-1R |
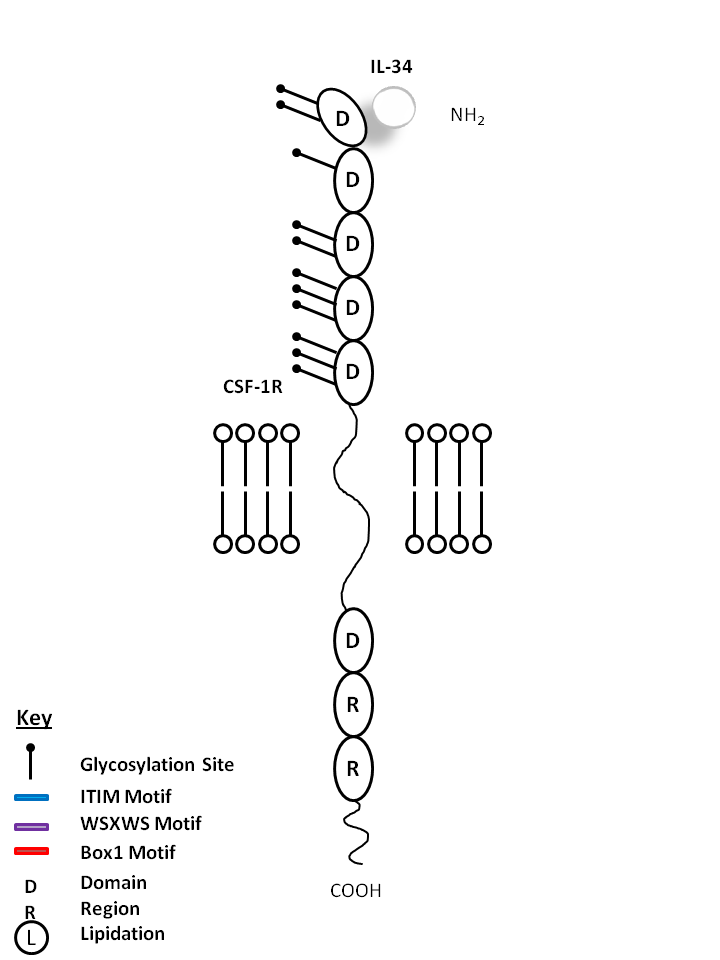 |
| IL-35 is bound by IL-12Rβ2 and gp130. IL-35 has been found to suppress T cell proliferation in vitro and promotes the development of iTregs35, a newly discovered subpopulation of Tregs |
| IL-35 | IL-12Rβ2 | gp130 |
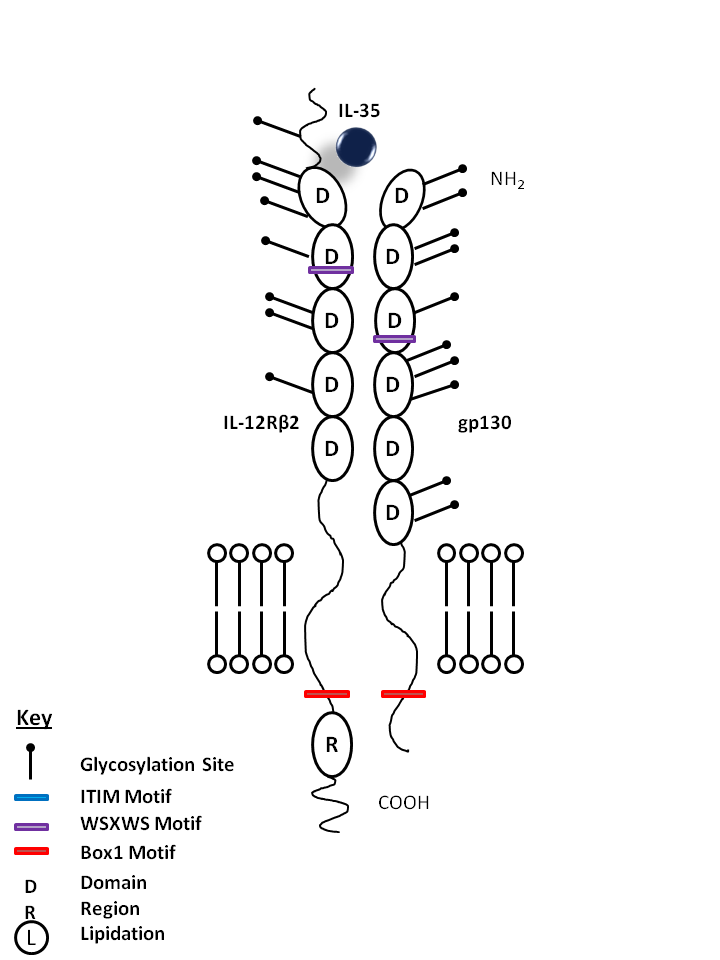 |
| IL-10 is bound by IL-10Rα and IL-10Rβ. It is crucial for the inhibition of antigen presentation and proinflammatory (Th1) responses. IL-10 also promotes a Th2 response, increasing antibody production. |
| IL-10 | IL-10Rα | IL-10Rβ |
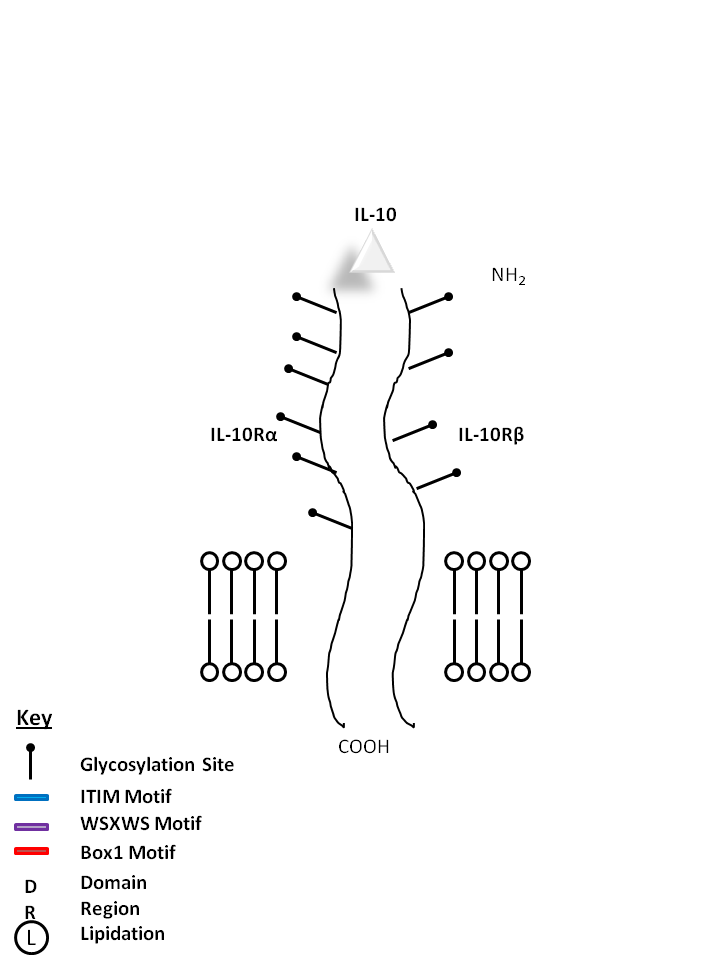 |
| IL-19, IL-20, and IL-24 can be bound by IL-29Rα and IL-20Rβ. IL-19 promotes Th2 phenotype development and also induces IL-6 and TNF-α production from monocytes. IL-20 may be involved with epidermal cell function and psoriasis. IL-24 has concentration-dependent functions. At low concentrations, it induces IFN-γ, IL-1β, IL-12, and TNF-α from monocytes. At high concentrations, it promotes apoptosis in tumor cells, but not normal ones. |
| IL-19 | IL-20 | IL-24 | IL-20Rα | IL-20Rβ |
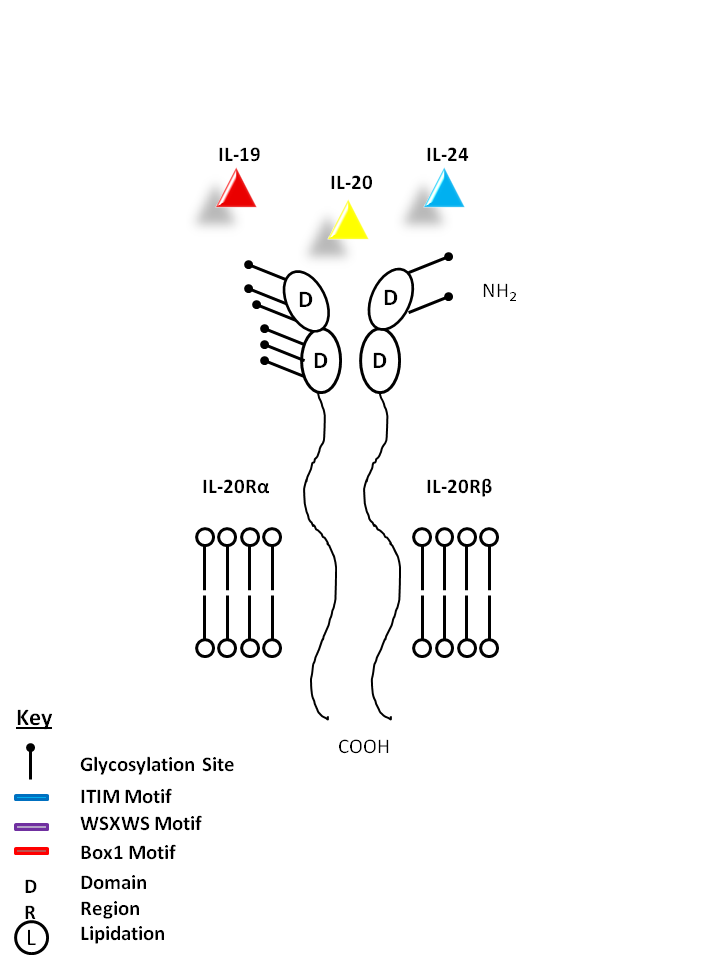 |
| IL-22 is bound by IL-22Rα1 and IL-10Rβ. Produced mainly by Th17 and Th22 cells, IL-22 plays a vital role in mucosal immunity and has been noted to promote Th17 transmigration across the blood brain barrier in multiple sclerosis. |
| IL-22 | IL-22Rα1 | IL-10Rβ |
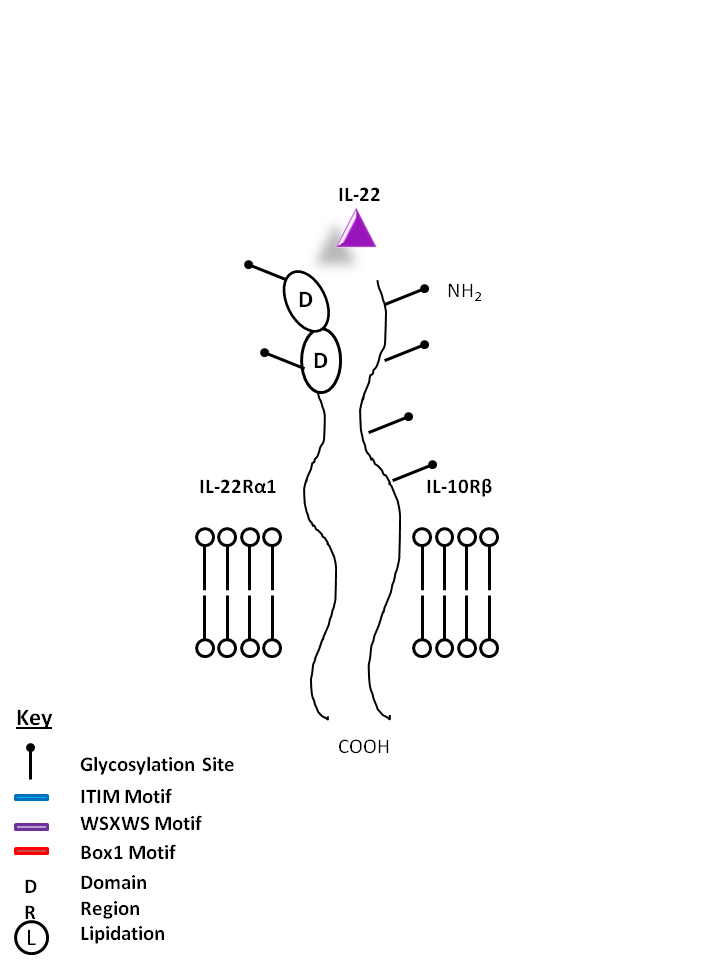 |
| IL-22 can also be bound by IL-22Rα2. This interaction antagonizes IL-22 function by binding to the cytokine, preventing its ligation to the receptor consisting of IL-22Rα1 and IL-10Rβ. |
| IL-22 | IL-22Rα2 |
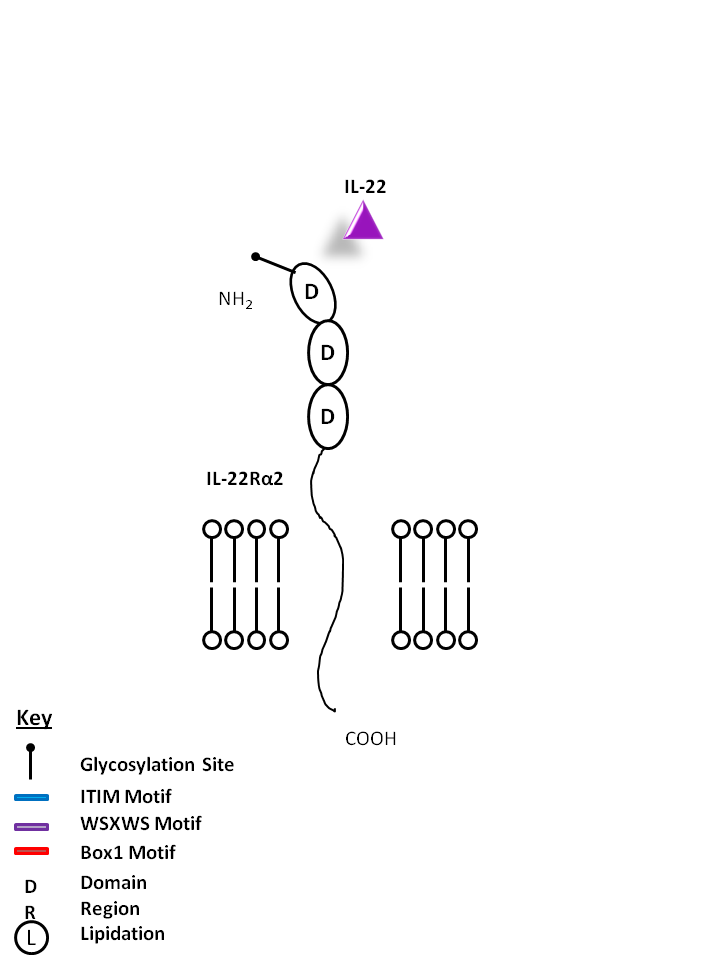 |
| IL-20 and IL-24 can be bound by IL-22Rα1 and IL-20Rβ. IL-20 may be involved with epidermal cell function and psoriasis. IL-24 has concentration-dependent functions. At low concentrations, it induces IFNγ, IL-1β, IL-12, and TNF-α from monocytes. At high concentrations, it promotes apoptosis in tumor cells, but not normal ones. |
| IL-20 | IL-24 | IL-22Rα2 | IL-20Rβ |
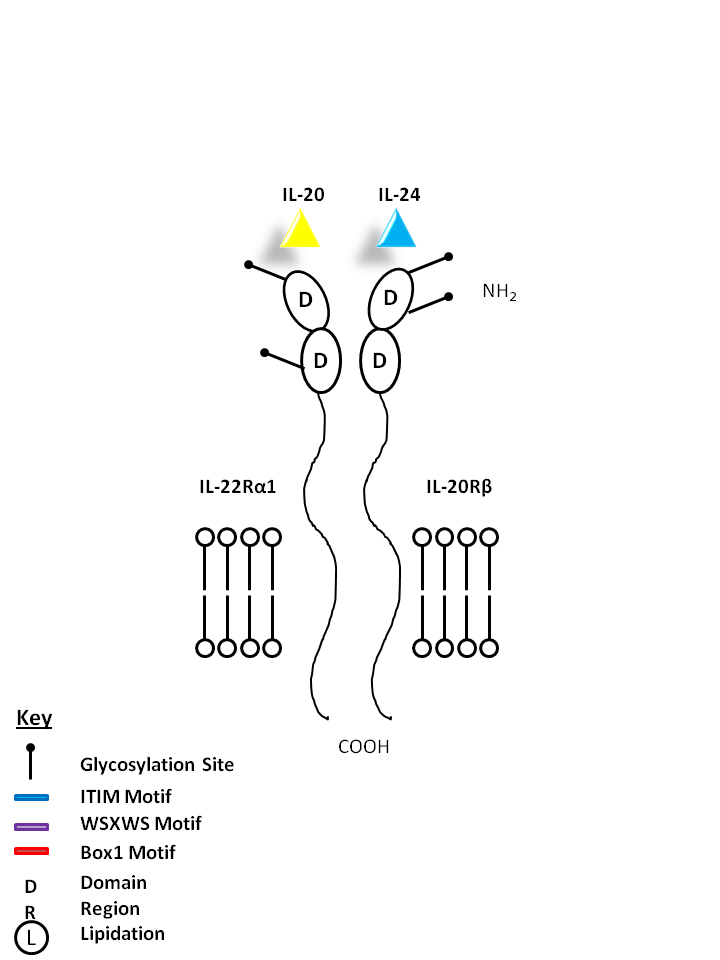 |
| IL-20 and IL-24 can be bound by IL-22Rα1 and IL-20Rβ. IL-20 may be involved with epidermal cell function and psoriasis. IL-24 has concentration-dependent functions. At low concentrations, it induces IFNγ, IL-1β, IL-12, and TNF-α from monocytes. At high concentrations, it promotes apoptosis in tumor cells, but not normal ones. |
| IL-26 | IL-20Rα | IL-10Rβ |
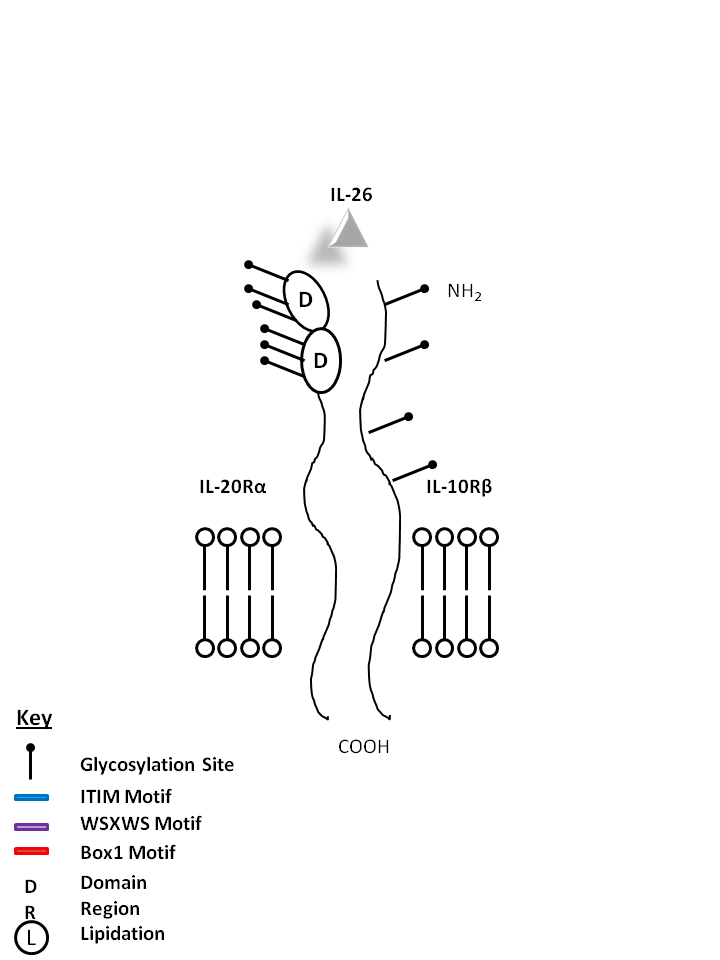 |
| IL-28A, IL-28B, and IL-29 can be bound by IL-28RA and IL-10Rβ. IL-28A, IL-28B, and IL-29 have similar function and are known to upregulate MHC I and display potent antiviral and antitumor activity. |
| IL-28A | IL-28B | IL-29 | IL-10Rβ | IL-28RA |
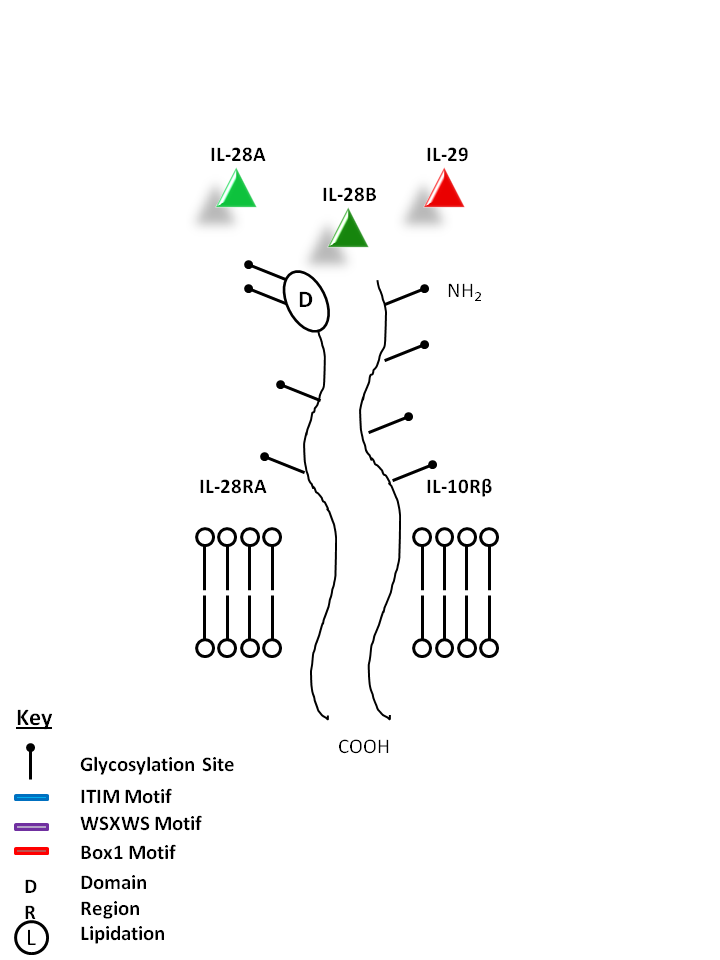 |
| Description |
| IL-1 is a pyrogen, and it is an activating factor for lymphocytes. It also damaged joints and influenced liver proteins. IL-1α binds to the cell surface type I and II IL-1 receptors (IL-1RI and IL-1RII). IL-1 and -β and IL-1RA can compete for binding to these receptors. However, only IL-1RI, not IL-1RII, is functional because IL-1RII lacks a cytoplasmic domain and is thus unable to transmit signals to downstream steps. IL-1α is expressed by cancer cells and promotes angiogenesis and metastasis of pancreatic cancer and human gastric cancer cell lines. In addition, IL-1 induced the expression of VEGF in colon cancer. |
| Distribution |
| Cellular Sources: Monocytes, tissue macrophages, Langerhans cells, dendritic cells, T and B cells, natural killer (NK) cells, large granular lymphocytes (LGL), vascular endothelium, smooth muscle, fibroblasts, thymic epithelia, astrocytes, microglia, glioma, keratinocytes; Cellular Targets: B cells, T cells, monocytes, dendritic cells. |
| View Products |
| Description |
| IL-1β in humans and mice does not encode a typical signal peptide and, as a result, newly synthesized pro-IL-1β accumulates within the cytoplasm of activated monocytes and macrophages. Conversion of the inactive pro-IL-1β to its mature form requires the proteolytic action of IL-1β-converting enzyme (ICE), also termed caspase-1. Secretion of mature IL-1β from LPS-activated monocytes/macrophages is not a constitutive process. These cells must encounter a secondary stimulus that specifically activates the posttranslational processing events. Moreover, owing to its pro-inflammatory nature, IL-1β is regarded as a tumor-promoting cytokine. In fact, enhanced tumor metastasis and angiogenesis has been observed under the influence of IL-1β. IL-1β is able to facilitate tumor progression in murine models of lung cancer. In addition, upregulation of metastasis and tumor angiogenesis by IL-1β has been associated with increased activity of matrix metalloproteinases and expression of the pro-angiogenic molecule hepatocyte growth factor. |
| Distribution |
| Cellular Sources: Monocytes, tissue macrophages, Langerhans cells, dendritic cells, T and B cells, natural killer (NK) cells, large granular lymphocytes (LGL), vascular endothelium, smooth muscle, fibroblasts, thymic epithelia, astrocytes, microglia, glioma, keratinocytes; Cellular Targets: B cells, T cells, monocytes. |
| View Products |
| Description |
| Interleukin-1 receptor antagonist (IL-1RA) is composed of three isoforms, one secreted (sIL-1Rα/IL-1Rα1), two intracellular (IL-1Rα2/IL-1Rα3) and produced by monocytes, tissue macrophages, Langerhans cells, dendritic cells, T and B cells, natural killer (NK) cells, large granular lymphocytes (LGL), vascular endothelium, smooth muscle, fibroblasts, thymic epithelia, astrocytes, microglia, glioma cells, keratinocytes, and chondrocytes. It is upregulated by IL-1β, IL-4, IL-6 and downregulated by glucocorticoids. It is a competitive inhibitor of IL-1 receptor binding and antagonizes IL-1 activities without having agonist effects. |
| Distribution |
| Cellular Sources: Monocytes, tissue macrophages, Langerhans cells, dendritic cells, T and B cells, natural killer (NK) cells, large granular lymphocytes (LGL), vascular endothelium, smooth muscle, fibroblasts, thymic epithelia, astrocytes, microglia, glioma, keratinocytes; Cellular Targets: B cells, T cells, monocytes. |
| View Products |
| Description |
| IL-1R1, also known as CD121a, is an IL-1 receptor type I. It is a transmembrane glycoprotein and a member of the immunoglobulin superfamily expressed on T cells, thymocytes, dendritic cells, fibroblasts, vascular endothelial cells, epithelial cells and neural cells. CD121a binds the mature forms of IL-1α, IL-1β, and IL-1Ra proteins. High affinity binding and signaling by the type I IL-1 receptor requires CD121a and the accessory protein, IL-1R AcP, which does not bind IL-1 alone, but forms a dimer capable of binding IL-1. |
| Distribution |
| T cells, thymocytes, dendritic cells, fibroblasts, vascular endothelial cells, epithelial cells, neural cells. |
| View Products |
| Description |
| IL-1R2, also known as CD121b, is an IL-1 receptor type II. It is a non-signaling receptor for IL-1α, IL-1β, and IL-1RA. It is mainly a decoy receptor that binds to its ligands to prevent interaction with Type 1 IL-1 receptors. This receptor can also be secreted with IL-1RAcP. |
| Distribution |
| T cells, thymocytes, dendritic cells, fibroblasts, vascular endothelial cells, epithelial cells, neural cells. |
| View Products |
| Description |
| Also known as IL-1R3, IL-1 Receptor Accessory Protein (IL-1RAcP) is a coreceptor that associates with IL-1R1 and IL-1R2. It is incapable of binding IL-1 alone. Secreted isoforms of IL-1RAcP also exist, increasing the affinity of IL-1R2 for IL-1β. |
| Distribution |
| T cells, thymocytes, dendritic cells, fibroblasts, vascular endothelial cells, epithelial cells, neural cells. |
| View Products |
| Description |
| IL-18, initially described as IFN-g inducing factor (IGIF), is a pro-inflammatory cytokine. It is identified as a costimulatory factor for production of interferon-γ (IFN-γ) in response to toxic shock and shares functional similarities with IL-12. It is reported that IL-18 is produced from Kupffer cells, activated macrophages, keratinocytes, intestinal epithelial cells, osteoblasts, adrenal cortex cells and murine diencephalon. The cytokine IL-18 possesses pleiotropic biological properties such as activation of NF-kb, Fas ligand expression, induction of both CC and CXC chemokines, enhancement of the production of IFN-g and GM-CSF, and induction of CD4+ cytolytic T cell response. Its role in neurodegeneartion disease has also been reported |
| Distribution |
| Cellular Sources: Monocytes, macrophages, dendritic cells, Kupffer cells, T cells, B cells, osteoblasts, epidermal keratinocytes, intestinal and corneal epithelial cells, astrocytes, microglial cells; Cellular Targets: Activated T cells, B cells, natural killer cells, neutrophils, endothelial cells, osteoclasts. |
| View Products |
| Description |
| IL-18RAcP is also known as CDw218b. In conjunction with IL-18Rα, it forms the high affinity complex for IL-18. It is strongly expressed in T-cells polarized with IL-12 and coexpressed with IL-18Rα. It is unable to bind IL-18 on its own. |
| Distribution |
| Expressed on NK cells and neutrophils, IL-12 activated T and B cells, endothelial cells, and smooth muscle cells, osteoclasts; highly expressed on Hodgkin's disease cell lines. |
| Description |
| IL-18 receptor is composed of an α and a β subunit that combine to form a high affinity receptor for IL-18. IL-18 receptor α chain, also known as CDw218a, is a type I transmembrane protein. It is expressed on NK cells, neutrophils, endothelial cells, and subsets of T and B cells. The expression of CDw218a on lymphocytes is upregulated after activation. The interaction of IL-18 and IL-18 receptor has been reported to be implicated in promotion of Th1 cytokine production and atherogenesis. |
| Distribution |
| Expressed on NK cells and neutrophils, IL-12 activated T and B cells, endothelial cells, and smooth muscle cells, osteoclasts; highly expressed on Hodgkin's disease cell lines. |
| View Products |
| Description |
| IL-33, also known as NFHEV and DVS 27, is a proinflammatory protein that belongs to IL-1 superfamily. IL-33 is constitutively expressed in smooth muscle and airway epithelia. It is upregulated in arterial smooth muscle, dermal fibroblasts, and keratinocytes following IL1α or IL1β stimulation. IL-33 interacts with IL1RL1/ST2 receptor and IL-1 receptor accessory protein (IL1RAP) in order to activate intracellular molecules in the NF-κB and MAP kinase signaling pathways that drive production of type 2 cytokines (e.g. IL-5 and IL-13) from polarized Th2 cells. Recombinant IL-33 has been shown to enhance Th2-associated immune responses and potently increase mast cell proliferation and cytokine production. |
| Distribution |
| Cellular Sources: High endothelial venules, smooth muscle cells, keratinocytes, epidermal cells, dermal fibroblasts, and osteoblasts. Macrophages, dendritic cells, iNKT, and NK cells may also express IL-33; Cellular Targets: Th2 cells, Eosinophils, dendritic cells , mast cells, basophils, Th2 cells, macrophages , neutrophils (mouse), iNKT cells, NK cells. |
| View Products |
| Description |
| IL-1RL1, also known as Interleukin-1 receptor-like 1 and ST2, forms a functional receptor for IL-33 with IL-1RAcP (IL-1 Receptor Accessory Protein). It is a member of the Toll-like receptor superfamily, signaling through MyD88, IRAK1, IRAK4, and IRAK6. It also activates several MAP kinases. IL-1RL1 is possibly involved with T helper cell function. A soluble form has been noted in fibroblasts and mast cells and has been shown to inhibit Th2 cytokine production. |
| Distribution |
| (Isoform A): T cells, basophils, eosinophils, mast cells, macrophages; (Isoform B): Epithelial cells, endothelial cells, fibroblasts, smooth muscle cells. |
| View Products |
| Description |
| IL-36α is also known as IL-1F6 and FIL1 epsilon. It shares homology and sequence similarity with IL-1α and IL-1β. Overexpression of IL-36α has been reported to cause skin abnormalities, and lesions and epithelial morphological changes in the kidneys of mice. IL-36α is the only reported IL-1 family member expressed on T cells. |
| Distribution |
| Cellular Sources: Monocytes, B cells, T cells, keratinocytes; Cellular Targets: Keratinocytes, adipocytes (human), bone marrow derived dendritic cells (mouse), CD4+ T cells (mouse). |
| View Products |
| Description |
| A member of the IL-1 family, IL-36β is also known as IL-1F8 and FIL-1H and IL-1H2. It has been found to stimulate production of IL-6 and IL-8 in synovial fibroblasts, articular chondrocytes, and mature adipocytes. It also induces the production of several antimicrobial peptides. |
| Distribution |
| Cellular Sources: Resting and activated monocytes, B cells; Cellular Targets: synovial fibroblasts, articular chondrocytes, mature adipocytes, bone marrow derived dendritic cells (mouse), CD4+ T cells (mouse). |
| View Products |
| Description |
| A member of the IL-1 family, IL-36γ is also known as IL-1F9 and IL-1H1. IL-36γ has been shown to signal through IL-1RAcP and IL-1RL2, activating NF-κB and MAPK pathways. |
| Distribution |
| Cellular Sources: Epithelial cells of the lung, stomach, and esophagus, keratinocytes, langerhans cells; Cellular Targets: Keratinocytes, neutrophils (mice), epithelial cells, bone marrow derived dendritic cells (mouse), CD4+ T cells (mouse). |
| View Products |
| Description |
| A member of the IL-1 family, IL-36RA is also known as FIL1 delta, IL-36RN, and IL-1F5. IL-36RA is a highly specific antagonist of the response of IL-1RL2 to IL-36γ and IL-36α. Defects in IL-36RA have been associated with psoriasis. |
| Distribution |
| Cellular Sources: Monocytes, B cells, dendritic cells/Langerhans cells, keratinocytes, epithelial cells, certain macrophage subsets; Cellular Targets: Keraitinocytes, epithelial cells, bone marrow derived dendritic cells (mouse), CD4+ T cells (mouse). |
| Description |
| IL-1RL2 is also known as Interleukin-1 Receptor-like 2 and Interleukin-1 receptor related protein-2 (IL-1Rrp2). It has been shown as incapable of binding IL-1α and IL-1β. In conjunction with IL-1RAcP, it serves as the receptor for IL-36. |
| Distribution |
| Expressed in a wide variety of tissues. In humans, highest expression is found in the skin, while the prostate, uterus, seminal vesicle, and paw show highest expression in mice. |
| Description |
| IL-37 is also known as IL1F7 (Interleukin-1 Family Member 7). IL-37 currently has five isoforms. IL-37 has been shown to bind to the IL-18 receptor, IL-18Rα and IL-18BP (IL-18Binding Protein). Unlike other members of the IL-1 cytokine family, IL-37 has been shown to have suppressive capabilities of innate inflammatory reactions (including IL-1A, IL-6, CSF1, CSF2, CCL12, CXCL13, IL-1B, and IL-23A). It has also been shown to inhibit dendritic cell activation. In general, there is low expression, if any, in healthy tissues. There is high expression in inflammatory counterparts. Testis, colon, placenta, lung, and lymph node have expressed Isoforms A, B, and C. Testis and bone marrow have expressed Isoforms D and E. Brain has only shown isoform A, kidney has only shown isoform B, and heart has only shown isoform C. |
| Distribution |
| Cellular Sources: Peripheral blood mononuclear cells, monocytes, plasma cells, tumor cells, epithelial cells; Cellular Targets: Hepatocytes, dendritic cells, monocytic and epithelial cell lines. |
| Description |
| IL-17A is the founding member of the IL-17 family, a group of six structurally related pro-inflammatory cytokines. IL-17A, secreted by activated CD4+ Th17 cell subpopulation, elicits multiple biological activities on a variety of cells including: the induction of IL-6, IL-8, G-CSF, and PGE2 production in epithelial, endothelial or fibroblasts; the enhancement of surface expression of ICAM-1 in fibroblasts; activation of NF-κB and costimulation of T cell proliferation. Recent studies demonstrated that, in mice, activated IL-17-secreting CD4+ helper T cells (Th17 cells)mediate an autoimmune arthritis that clinically and immunologically resembles rheumatoid arthritis (RA). Human IL-17A shows 63%, 63% and 72% amino acid sequence identity to rat IL-17A, mouse IL-17A and a protein encoded by the ORF13 gene of herpesvirus Saimiri (HVS), respectively. Both recombinant and natural IL-17A have been shown to exist as a disulfide-linked homodimeric glycoprotein containing two ~16 kD monomers. |
| Distribution |
| Cellular Sources: activated memory T lymphocytes, CD4+ T helper cells (Th17), neutrophils, CD8(+), NK, and gamma-delta T cells; Cellular Targets: Fibroblasts, epithelial and endothelial cells, stromal cells. |
| View Products |
| Description |
| Six interleukin-17 (IL-17)-family (IL-17A-IL-17F) and five IL-17 receptor (IL-17R)-family (IL-17RA-IL-17RE) members have been reported in mammalian cells. IL-17RA (known as IL-17R, IL-17AR, CD217) is a 128-158 kD type I transmembrane glycoprotein. IL-17R binds to IL-17A (also known as IL-17, and CTLA-8, is a hallmarker of Th17 cells) and IL-17F and mediates the proinflammatory signals through interaction with IL-17RC or IL-17RD. IL-17R has broad tissue distribution, including fibroblasts, epithelial cells, endothelial cells, stromal cells, B cells, T cells, NK cells, monocytes and granulocytes. |
| Distribution |
| Broad tissue distribution, including fibroblasts, epithelial cells, endothelial cells, stromal cells, B cells, T cells, NK cells, monocytes and granulocytes. |
| View Products |
| Description |
| Also known as SEF, IL-17 RD is known to exist in several isoforms. IL-17 RD has been shown to form homomultimeric complexes. It can also complex with IL-17RA. Aside from IL-17A, no other ligands for these receptors have been identified. IL-17RD has been shown to be a feedback inhibitor of fibroblast growth factor mediated Ras-MAPK signaling and ERK activation. It may also mediate apoptosis, proliferation, migration, and angiogenesis. |
| Distribution |
| Endothelial cells, Epithelial cells. |
| Description |
| IL-17B is a protein also known as Cytokine CX1, NIRF, and Zcyto7. Originally referred to as IL-20, IL-17B is capable of stimulating the release of TNFα and IL-1β from a monocytic cell line. It is also known to promote proinflammatory responses. |
| Distribution |
| Cellular Sources: Cells of the gastrointestinal tract, pancreas, neurons, chondrocytes; Cellular Targets: Endothelial cells, neutrophils, keratinocytes. |
| View Products |
| Description |
| Also known as IL-25R, IL-17RB is a receptor for IL-17B and IL-17E (IL-25). It forms a homodimer for the binding of IL-17B, while forming a heterodimer with IL-17RA to serve as the receptor for IL-17E. It may also play a role in controlling the growth and/or differentiation of hematopoietic cells. IL-17RB has been shown to activate NF-κB and lead to the production of IL-8 (due to IL-17E binding). IL-17RB expression is strongly correlated with a Th2 response. |
| Distribution |
| Activated T cells, endothelial cells, endocrine tissue, macrophages, NKT cells (mice), CD4+ iNKT cells (mice), dendritic cells (human), basophils (human). |
| View Products |
| Description |
| IL-17C is a protein and a T cell-derived cytokine that shares the sequence similarity with IL-17. This cytokine was reported to stimulate the release of TNFα and IL-1β from a monocytic cell line. The expression of this cytokine was found to be very limited (adult prostate and fetal kidney) and restricted to activated T cells. |
| Distribution |
| Cellular Sources: Activated T cells, epithelial cells, cells of the prostate, lung, skin and fetal kidney; Cellular Targets: Epithelial cells. |
| Description |
| IL-17RE exists in multiple isoforms that can exist as a single-pass type 1 membrane protein, in a secreted form, or in the cytoplasm. IL-17RE binds with high affinity to IL-17C, but not IL1-7A, IL-17B, IL-17E, or IL-17F. Recently, it has been found to combine with IL-17RA to bind IL-17C. Combined with IL-17RA, this receptor is known to induce the production of anti-bacterial peptides and proinflammatory molecules. |
| Distribution |
| T cell subsets, epithelial cells of the lungs, gastrointestinal tract, skin, and lungs. |
| Description |
| IL-17D is a protein known to induce IL-6, IL-8, and GM-CSF production from endothelial cells. It is found to be preferentially expressed in skeletal muscle, brain, adipose, heart, lung, and pancreas tissues. Its receptors are currently unknown. |
| Distribution |
| Cellular Sources: Cells of the muscles, brain, heart, lung, pancreas and adipose tissue; Cellular Targets: Endothelial cells, myeloid progenitor cells. |
| Description |
| IL-25 (IL-17E) is a member of the IL-17 family of proinflammatory cytokines which include at least six members including IL-17A (IL-17), IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25), and IL-17F. IL-17E is found at low levels in various peripheral tissues. It is a proinflammatory cytokine that promotes airway eosinophilia in mice. IL-17E plays a role in Th2 type inflammatory diseases, such as asthma and atopic dermatitis. |
| Distribution |
| Cellular Sources: Activated Th2 cells, bone marrow-derived mast cells, and alveolar macrophages , microglia; Cellular Targets: CD4+ NKT cells, macrophages, endothelial cells, Th2, and Th9 cells. |
| View Products |
| Description |
| IL-17A/F is a heterodimer of IL-17A and IL-17F, two of the most studied members of the IL-17 family, consisting of at least six proinflammatory cytokines. Among the IL-17 family members, IL-17A and IL-17F share the highest sequence homology of 50%, and they are located on the same chromosome region. Earlier studies have shown that both IL-17A and IL-17F are produced as homodimers by Th17 cells, an activated subset of CD4+ T cells. However, recent studies demonstrate that the IL-17A/F heterodimer is also produced in Th17 cells, as a 40 kD, secreted, disulfide-linked glycoprotein. Functionally, IL-17A/F heterodimers signal through the same receptor as IL-17A and IL-17F homodimers and induce similar biological responses. However, both mouse and human IL-17A demonstrate more than 100-fold higher biological activity than IL-17F, while IL- 17A/F has intermediate activity. Both IL-17A and IL-17F have been associated with many inflammatory diseases in humans such as rheumatoid arthritis, multiple sclerosis, psoriasis, inflammatory bowel disease, and asthma. The exact cellular functions of IL-17A/F and its role in the disease processes are still not very clear. A convenient and ready-to-use IL-17A/F kit will be a very useful tool for those involved in the IL-17A, IL-17F, and IL-17A/F research efforts. |
| Distribution |
| Cellular Sources: activated memory T lymphocytes, CD4+ T helper cells (Th17), neutrophils, CD8(+), NK, and gamma-delta T cells; Cellular Targets: Fibroblasts, epithelial and endothelial cells, stromal cells. |
| View Products |
| Description |
| IL-17F belongs to the IL-17 cytokine family which includes IL-17A, B, C, D, and E (also called IL-25). IL-17F shares the strongest homology to IL-17A. They share 50% amino acid sequence homology. The genes encoding IL-17 and IL-17F are localized in the same chromosomal region and are co-expressed by CD4+ and gamma delta T cells. Recently, an IL-17-IL-17F heterodimer was found to be expressed in Th17 cells together with IL-17 and IL-17F homodimers. Similar to IL-17, IL-17F utilizes IL-17RA and IL-17RC as its receptor and employs Act1 and TRAF6 as its signal transducers to induce the expression of pro-inflammatory cytokines and chemokines in many different cell types. IL-17F expression is upregulated in inflammatory bowel disease. A His161 to Arg161 (H161R) substitution in the third exon of the IL17F gene seems to be associated with asthma and chronic obstructive pulmonary disease (COPD) in Japanese subjects. In addition polymorphism of IL-17F seems to be associated to susceptibility to gastric cancer. |
| Distribution |
| Cellular Sources: T cells, Th17 cells, memory CD4 T cells, gamma delta T cells, activated monocytes; Cellular Targets: keratinocytes, fibroblasts, macrophages, neutrophils, epithelial cells, activated human mast cells, and basophils. |
| View Products |
| Description |
| IL-17RC is a single pass transmembrane, 86kD protein that shares limited similarity with the interleukin 17 receptor. It is widely expressed in non leukocyte tissues and forms a heteromeric receptor for IL17 with IL-17RA. At least four different isoforms (ranging from 59 to 86kD) are known to exist. IL-17RC is expressed in heart, brain, intestine, liver, kidney, lung, muscle, placenta and prostate. Although IL-17RC is capable of binding to IL-17A, IL-17F, and IL-17A/F, IL-17RA is required for proper signaling. |
| Distribution |
| Broad tissue distribution, including epithelial cells, fibroblasts, Stromal cells, B cells, T cells, monocytes, NK cells. |
| Description |
| IL-2 was discovered through its function as a T cell growth factor (TCGF), and plays a pivotal role in immune responses against pathogenic infection. Recognition and binding of the foreign Ags by the TCRs stimulate both the secretion of IL-2 and the expression of IL-2Rs on the T cell surface. Subsequently, the IL-2/IL-2R interaction activates the intracellular Ras/Raf/MAPK, JAK/STAT, and PI3K/AKT signal pathways, and ultimately stimulates the growth, differentiation, and survival of the Ag-selected cytotoxic T cells. |
| Distribution |
| Cellular Sources: T cells; Cellular Targets: T cells, B cells, NK cells, LAK cells, monocytes, macrophages, oligodendrocytes. |
| View Products |
| Description |
| The high affinity IL-2 receptor consists of IL-2Rα, IL-2Rβ, and IL-2Rγ. IL-2Rα, also known as CD25, is a glycoprotein capable of binding to IL-2 with low affinity but cannot initiate a signal. Reports have also shown soluble forms of IL-2Rα, but its function is unknown. |
| Distribution |
| Activated T and B cells, thymocyte subset, pre-B cells, T regulatory cells , activated monocytes/macrophages. |
| View Products |
| Description |
| CD132 is a type I transmembrane glycoprotein of the Ig superfamily, also known as common γ chain (γc), or IL-2 receptor γ subunit. It is a member of the intermediate and high affinity IL-2 receptor complexes. It is expressed broadly on T- and B-lymphocytes, NK cells, monocytes, and granulocytes. CD132 is an essential component of cytokine receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. Ligand binding induces tyrosine phosphorylation and initiates signaling through a JAK/STAT pathway. CD132 mutation results in X-linked severe combined immune deficiency (XSCID). |
| Distribution |
| T, B, NK, monocytes, granulocytes. |
| View Products |
| Description |
| CD122 is an IL-2 receptor β chain also known as IL-2Rβ, which is also shared by the IL-15 receptor. It is a member of the intermediate and high affinity IL-2 receptor complexes. It is constitutively expressed by NK cells and at lower levels by T cells, B cells, monocytes, and macrophages. The IL-2Rβ chain can combine with either the common γ subunit (γc, CD132) alone or with the γc subunit and the IL-2Rα subunit (CD25) to generate intermediate or high affinity IL-2 receptor complexes, respectively. CD122 expression levels can be upregulated by activation. |
| Distribution |
| NK Cells, T cells, B cells, Monocytes, Macrophages. |
| View Products |
| Description |
| IL-3 is a highly species-specific pleiotropic factor produced primarily by activated T cells though also by mast cells keratinocytes, and astrocytes, which stimulates colony formation of megakaryocytes, neutrophils, and macrophages from bone marrow cultures. |
| Distribution |
| Cellular Sources: Activated T cells, mast cells, eosinophils, keratinocytes, NK cells, endothelial cells ; Cellular Targets: Erythroid cells, megakaryocytes, neutrophils, eosinophils, basophils, mast cells, monocytic lineages. |
| View Products |
| Description |
| CD123 is the transmembrane α chain of the IL-3 receptor. Alone, CD123 binds IL-3 with low affinity; when CD123 associates with CDw131 (common β chain), it binds IL-3 with high affinity. CD123 does not transduce intracellular signals upon binding IL-3 and requires the β chain for this function. CD123 is expressed by myeloid precursors, macrophages, dendritic cells, mast cells, basophils, megakaryocytes, and some B cells. |
| Distribution |
| Hematopoietic progenitors (mouse), basophils, mast cells, megakaryocytes , myeloid precursors (human), macrophages (human), dendritic cells (human). |
| View Products |
| Description |
| CD131, also known as the IL-3R common β subunit (βC), is a type I transmembrane glycoprotein and belongs to the Ig superfamily. The common β subunit associates with the specific α subunits of IL-3 receptor, IL-5 receptor and GM-CSF receptor to form high affinity receptors for these cytokines. These cytokine receptors are expressed by neutrophils, eosinophils, monocytes, endothelial cells, fibroblasts and hematopoietic progenitor cells and play a crucial role in growth/activation of eosinophils and in the inflammatory response. |
| Distribution |
| Low levels on monocytes, granulocytes, eosinophils, basophils, hematopoietic progenitors, endothelial cells. |
| View Products |
| Description |
| IL-4 is the primary cytokine implicated in the development of Th2-mediated responses, which is associated with allergy and asthma. The Type I receptor comprises IL-4Rα and the common gamma-chain (γc), which is also shared by the cytokines IL-2, -7, -9, -15 and -21 and is present in hematopoietic cells. IL-4 can use the type II complex, comprising IL-4Rα and IL-13Rα1, which is present in non-hematopoietic cells. This second receptor complex is a functional receptor for IL-13, which shares approximately 25% homology with IL-4. The type I receptor complex can be formed only by IL-4 and is active in Th2 development. In contrast, the type II receptor complex formed by either IL-4 or IL-13 is most active during airway hypersensitivity and mucus secretion and is not found in T cells. |
| Distribution |
| Cellular Sources: Subsets of naïve CD4+ T cells, Th2 cells, NK T cells, mast cells, basophils, eosinophils, and macrophages; Cellular Targets: B cells, T cells, monocytes, endothelial cells, and fibroblasts. |
| View Products |
| Description |
| IL-4R is also known as CD124. It is known to bind IL-4 in combination with the common gamma chain (IL-2Rγ) or IL-13Rα. It is also known to bind IL-13 when joined with IL-13Rα. A soluble isoform of IL-4R is known to inhibit both IL-4 mediated cell proliferation and upregulation of IL-5 by T cells. |
| Distribution |
| Activated T cells. |
| View Products |
| Description |
| Also known as CD213a1, IL-13Rα1 is a protein capable of binding to IL-13 when paired with IL-4R. This pairing also lets it serve as an alternate for the binding of IL-4. |
| Distribution |
| B cells, T cells, Endothelial Cells. |
| View Products |
| Description |
| IL-5 is a homodimeric, disulphide-linked protein produced by T-cells. Mouse and human IL-5 are approximately 70% identical. IL-5 has been shown to promote the growth of immature hematopoietic BFU-E progenitors, stimulate the activation and differentiation of eosinophils, and promote the generation of cytotoxic lymphocytes. |
| Distribution |
| Cellular Sources: Mast cells, T cells, eosinophils, bone marrow stromal cells; Cellular Targets: Eosinophils, B cells. |
| View Products |
| Description |
| IL-5Rα is also known as CD125 and exists in membrane bound and soluble forms. In conjunction with CSF2RB (CD131/common β subunit), it serves as the receptor for IL-5. |
| Distribution |
| Eosinophils, basophils, B cells (mice). |
| View Products |
| Description |
| IL-6 is a potent lymphoid cell growth factor that stimulates the growth and survival of certain B cells and T cells. IL-6 plays a role in host defense, acute phase reactions, immune response, and hematopoiesis. IL-6 is expressed by T cells, B cells, monocytes, fibroblasts, hepatocytes, endothelial cells and keratinocytes. |
| Distribution |
| Eosinophils, basophils, B cells (mice). |
| View Products |
| Description |
| CD126 is also known as IL-6R. It is a member of the immunoglobulin superfamily that is expressed on plasma cells, T cells, activated B cells, monocytes, hepatocytes, epithelial cells, and fibroblasts. Functional IL-6 receptors are formed by the non-covalent association of CD126 and the IL-6 receptor β chain (CD130 or gp130). CD126 binds IL-6 with low affinity, but does not signal. The β chain (gp130, CD130) does not bind IL-6 by itself, but associates with the α-chain/IL-6 complex to initiate signal transduction. IL-6 binding to the receptor complex results in the stimulation of B and T cells, and hematopoietic precursor proliferation and differentiation. Soluble form of CD126 has been found in human serum. |
| Distribution |
| Activated T and B cells, monocytes, hepatocytes, plasma cells. |
| View Products |
| Description |
| gp130 is also known as CDw130 and Interleukin-6 signal transducer. gp130 is a 103kD protein common to several cytokine receptors, such as IL-6, IL-11, IL-27, LIF, OSM, and BSF3.Typically, gp130 is utilized for the initiation of signal transmission. Smaller, soluble isoforms have been discovered. |
| Distribution |
| T cells, Activated B cells, plasma cells, monocytes, endothelial cells. |
| View Products |
| Description |
| IL-7 was initially described as a stromal derived factor which is capable of inducing the growth of pre-B cells in vitro. The generation of IL-7 deficient and IL-7Rα deficient mice, and monoclonal antibody blocking experiments confirmed the requirement of IL-7 for B-cell development in mice. Mutations in the a chain of the IL-7 receptor in patients with severe combined immunodeficiency (SCID) confirmed that IL-7 is indispensable for T-cell development in humans. However, the presence of B cells in these individuals suggests important differences between the role of IL-7 in murine and human lymphocyte development. Thus, although human B-cell development does not appear to require IL-7, immature human B cells do proliferate in response to it. New information suggests that IL-7 dependence in human lymphopoiesis increases during the progression of ontogeny in cord blood and bone marrow. IL-7 can associate with hepatocyte grow factor (HGFβ) to form a hybrid cytokine (IL-7/HGFβ), which induces greater proliferation of CFU-S, SLPs, and pre-pro-B cells than native IL-7. The hybrid cytokine signals through both IL-7R (IL-7Rα plus γc) and c-Met. IL-7 has a thymic antiapoptotic effect and induces the expression of antiapoptotic proteins Bcl-2 and Mcl-1 and the inhibition of proapoptotic proteins Bax and Bad. In addition, IL-7 is a key regulator of glucose uptake in T lymphocytes. TGF-β has been shown to down-regulate IL-7 mRNA and protein secretion from human bone marrow stromal cells. In addition, TGF-β inhibits IL-7-induced proliferation of pre-B cells. |
| Distribution |
| Cellular Sources: Epithelial and stromal cells in thymus, bone marrow, epithelial cells of the intestine, epithelial goblet cells, keratinocytes, fetal liver, adult liver, dendritic cells, skeletal muscle cells (human), fibroblastic reticular cells, and follicular dendritic cells, endothelial cells; Cellular Targets: Progenitor B and T cells, CD4-CD8- thymocytes, megakaryocytes. |
| View Products |
| Description |
| CD127 is a type I transmembrane glycoprotein also known as IL-7 receptor α chain or IL-7Rα. It forms a heterodimer with the common γ chain (γc or CD132) which is shared with the receptors for IL-2, IL-4, IL-9, IL-13, IL-15, and IL-21. CD127 is expressed on immature B through early pre-B stage cells, thymocytes (except CD4/CD8 double positive thymocytes), peripheral T cells, and bone marrow stromal cells. CD127 has been reported to be a useful marker for identifying memory and effector T cells. Recent studies have shown that CD127 expression is down-modulated on Treg cells. It can be used as a marker for differentiation of Treg and conventional T cells. The ligation of IL-7 with its receptor is important for stimulation of mature and immature T cells as well as immature B cell proliferation and development. |
| Distribution |
| Immature B cells through early pre-B stage, thymocytes (except CD4/CD8 double positive thymocytes), peripheral T cells, bone marrow stromal cells. |
| View Products |
| Description |
| IL-9 is a potent, T cell-derived, T cell growth factor which can also enhance mast cell activity and IL-3- or IL-4- dependent proliferation of bone marrow-derived mast cells. IL-9 synergizes with erythropoietin to promote erythroid colony formation. IL-9 has also been reported to protect human T cells from apoptosis induced by IL-2 withdrawal. IL-9 is upregulated in human eosinophils by TNF-α and IL1-β. IL-9 has been reported to downregulate the oxidative burst in activated human alveolar macropahges and induce TGF-β production. In mice, IL-9 induces high affinity IgE receptor expression and granzyme A and B and T helper clones. |
| Distribution |
| Cellular Sources: IL-2 activated TH2 lymphocytes, Hodgkin's lymphoma ; Cellular Targets: T lymphocytes, erythroid precursors. |
| View Products |
| Description |
| CD129 is known as the IL-9 receptor. It is a member of the hematopoietin receptor superfamily. Although the α-chain of the receptor binds IL-9 with high affinity, interaction with the γ-chain (CD132) of the IL-2 receptor is required for signaling. The IL-9 receptor is expressed at low levels on eosinophils, mast cells, macrophages, B lymphocytes, T lymphocytes, and erythroid progenitors. IL-9 receptor binding initiates STAT activation required for the proliferative and anti-apoptotic effects of this cytokine. In humans, signals from the IL-9 receptor appear to be critical for intrathymic T cell development. IL-9 binding has been shown to increase IL-5 receptor expression and promote survival in human eosinophils. |
| Distribution |
| Eosinophils, mast, thymocytes, erythroid, myeloid progenitors, macrophages. |
| View Products |
| Description |
| IL-11 is a 21 kD cytokine directly involved with stimulating the proliferation of hematopoietic stem cells and megakaryocyte progenitor cells, inducing maturation and resulting in platelet production. IL-11 has also been shown to be involved with regulating B cell function, the induction of acute phase proteins from hepatocytes, and osteoclast development. |
| Distribution |
| Cellular Sources: Stromal Cells, Epithelial Cells, Fibroblasts, and Smooth Muscle Cells; Cellular Targets: T cells, B cells, hematopoeitic stem cells, megakaryocyte progenitor cells. |
| View Products |
| Description |
| IL-11Rα is a protein that associates with gp130 to form a high affinity receptor for IL-11. This complex is involved with proliferation and differentiation of skeletogenic progenitors and mesenchymal stem cells. It is also involved with the development of bones in the skull and teeth and is upregulated in certain cancer cell lines. |
| Distribution |
| Hematopoietic Stem Cells, Megakaryocyte Progenitors, Epithelial Cells, and Stromal cells. |
| Description |
| IL12 (p70) is a disulfide-linked heterodimer composed of unrelated p40 (glycosylated) and p35 subunits. IL-12 acts as a growth factor for activated human T and NK cells, enhances the lytic activity of human NK cells, and stimulates the production of IFNg by resting human PBMC. IL-12R is formed by two chains, IL-12Rβ1 and IL-12Rβ2. IL-12Rβ1 is associated with the Janus kinase (Jak) Tyk2 and binds IL-12 p40; IL-12Rβ2 is associated with Jak2 and binds either the heterodimer or the p35 chain. Signaling through the IL-12 receptor complex induces phosphorylation, dimerization, and nuclear translocation of several signal transducers and activators of transcription (STAT) family members (STAT1, 3, 4, 5), but most of the biological responses to IL-12 have been attributed to STAT4. IL-12 has been shown to elicit anti-tumor activity in mice and humans. It is believed that the antitumor effects of IL-12 are mediated, at least in part, by indirect mechanisms. Induction of IFN-γ results in the upregulation of class I and class II MHC molecules, adhesion molecules (ICAM-1), nitric oxide production by antigen presenting cells (APC), and the production of additional cytokines, CXCL9 and 10, which in turn mediate angiostatic effects; Cytokine detection: IL-12, IL-23 and IL-35 share common subunits, utilizing combinations of p40, p19 and p35 proteins. Caution must be used when selecting antibodies and assays when specific identification, measurement, as well as activation state discrimination, is required. 1. Active IL-12 consists of two subunits: p40 + p35. 2. p40 can also exist as a monomer (IL-12 p40) or a homodimer (IL-12 p80). 3. Besides contributing to IL-12, p40 is also found in IL-23, a heterodimer of p40 and p19. 4. Similarly, p35 not only contributes to IL-12, but is also found in the heterodimer IL-35 (p35 and EBI3). Note that assays using antibodies specific for the p40 subunit will be unable to discriminate between the active IL-12, monomeric p40, dimeric p40, and IL-23; likewise, assays using p35 detection alone will pick up both IL-12 (heterodimer) and IL-35. |
| Distribution |
| Cellular Sources: Dendritic cells, B cells, T cells Cellular Sources: Dendritic cells, B cells, T cells; Cellular Targets: T cells, NK cells, peripheral blood lymphocytes. |
| View Products |
| Description |
| IL-12Rβ1 is also known as CD212. It functions as an receptor which binds interleukin-12 with low affinity and is involved in IL12 transduction. When associated with IL12RB2, it forms a functional, high affinity receptor for IL12. It can also associate with IL23R to form the interleukin-23 receptor, which likely signals through the JAK-STAT pathway. |
| Distribution |
| Activated T cells, B cells, NK cells, certain subsets of Dendritic cells. |
| Description |
| Both mouse and human IL-13 were initially cloned from cDNA libraries of their respective activated T cells. IL-13 is an immunoregulatory cytokine secreted predominantly by activated T(H)2 cells, and it is a key mediator in the pathogenesis of allergic inflammation. IL-13 shares many functional properties with IL-4, and they share a common receptor subunit, the alpha subunit of the IL-4 receptor (IL-4Ralpha). IL-13 mediates its effects by interacting with a complex receptor system comprised of IL-4Ralpha and two IL-13 binding proteins, IL-13Ralpha1 and IL-13Ralpha2. Ligation of the IL-13 receptor complex results in signaling via the insulin receptor substrate (IRS)-1 and 2 and STAD-6 pathways. Interleukin-13 (IL-13), like IL-4, is a cytokine produced by T(H)2 type helper T cells in response to signaling through the T cell antigen receptor and by mast cells and basophils upon cross-linkage of the high-affinity receptor for immunoglobulin E (IgE). IL-13 has been implicated in airway hypersensitivity and mucus hypersecretion, inflammatory bowel disease, and parasitic nematode expulsion. |
| Distribution |
| Cellular Sources: Activated T helper cells, CD8+ T cells, mast cells, NK cells; Cellular Targets: B cells, monocytes, macrophages, basophils. |
| View Products |
| Description |
| CD213a2, also known as IL-13Rα2, is a type I transmembrane protein and member of the hematopoietin family. IL-13Rα2 is the high affinity receptor for IL-13, but possibly acts as a decoy receptor to antagonize IL-13Rα1/IL-4Ra-mediated IL-13 biological functions. IL-13Rα2 is expressed by fibroblasts, smooth muscle cells, keratinocytes, and activated B cells. However, its presence on the cell surface is highly regulated. CD213a2 is also expressed in various types of cancer which makes it an attractive therapeutic target. |
| Distribution |
| Fibroblasts, smooth muscle cells, keratinocytes, activated B cells. |
| View Products |
| Description |
| IL-15 was discovered in the supernatant from a simian kidney epithelial cell line CV-1/EBNA, as a soluble factor capable of supporting proliferation of the IL-2-dependent cell line, CTLL-2. Interleukin-15 (IL-15) is a regulatory cytokine, and it is produced by dendritic cells, epithelial cells, human stromal cell line (IMTLH), fibroblasts, and monocytes. IL-15 plays an important role in immune response and shares many functions with IL-2, for example, stimulating the proliferation of activated T cells, NK cells and B cells, and inducing immunoglobulin synthesis by B cells stimulated by anti-IgM or CD40 ligand. In addition, IL-15 promotes the development of dendritic cells, activates human neutrophils and induces the production of proinflammatory cytokines from macrophages. IL-15 acts as a bridge between innate and adaptive immunity because of its diverse roles in the immune system. IL-15 binds to heterotrimeric receptors composed of IL-15Rα, IL-15Rβ, and IL-15Rγc. IL-15 shares with IL-2 the receptor chains β and γc. IL-15 is normally not secreted in soluble form but is held on the cell surface bound to a unique receptor, IL-15Rα, especially on dendritic cells. Cell-bound IL-15 then is presented in trans to T cells and NK cells and is recognized by the γc receptor on these cells; such recognition maintains cell survival and intermittent proliferation. |
| Distribution |
| Cellular Sources: adherent peripheral blood mononuclear cells, fibroblasts and epithelial cells; Cellular Targets: T lymphocytes, NK cells. |
| View Products |
| Description |
| Also known as CD215, IL-15 receptor α (IL-15Rα) is a cytokine receptor with the structural homology to IL-2Rα (CD25). Both α receptors contain a group of β sandwich protein called sushi domains. Similar to IL-2Rα, IL-15Rα is able to form heterotrimer with their β chain (CD122) and common γ chain (γc, CD132). But unlike IL-2Rα subunit, IL-15Rα itself binds to IL-15 with equivalent high affinity comparing with the heterotrimeric complex. IL-15Rα is expressed as soluble or transmembrane forms by variety cells, such as activated monocytes, dendritic cells, CD8+ memory T cells, NK or NKT cells. IL-15Rα by itself forms stable complexes with IL-15 on cell surface and presents IL-15 in trans to neighboring cells. IL-15, like IL-2, is a pleiotropic four-helix bundle cytokine and plays a key role in promoting T and B cell survival and proliferation, preferentially in memory CD8+ T cell proliferation, and NK/NKT cell development. |
| Distribution |
| Dendritic cells, activated monocytes, memory CD8 T cells and NK/NKT cells. |
| View Products |
| Description |
| Interleukin-16 is a proinflammatory cytokine synthesized as a 68 kD precursor molecule (pro-IL-16). After cleavage of pro-IL-16 by caspase-3, IL-16 is released as a 12 kD protein, but its biologic activities are exerted by a tetramer. IL-16 is a chemoattractant for CD4+ T cells, monocytes, and eosinophils. On T cells, IL-16 upregulates CD25 and class II expression, and also inhibits HIV-1 replication in vitro by repressing the transcription of the HIV-1 long terminal repeat. IL-16 is expressed by lymphocytes, macrophages, eosinophils, mast cells, epithelial cells, and fibroblasts. CD4 and CD9 are the receptors of IL-16. |
| Distribution |
| Cellular Sources: Lymphocytes, macrophages, eosinophils, mast cells, epithelial cells, and fibroblasts; Cellular Targets: CD4+ T cells, monocytes, and eosinophils. |
| View Products |
| Description |
| CD4, also known as T4, is a single-chain type I transmembrane glycoprotein expressed on most thymocytes, a subset of T cells, and monocytes/macrophages. CD4, a member of the Ig superfamily, recognizes antigens associated with MHC class II molecules and participates in cell-cell interactions, thymic differentiation, and signal transduction. CD4 acts as a primary receptor for HIV, binding to HIV gp120. CD4 has also been shown to interact with IL-16. |
| Distribution |
| T cell subset, majority of thymocytes, monocytes/macrophages. |
| View Products |
| Description |
| CD9 is a type III transmembrane protein also known as tetraspanin, MRP-1 and DRAP-24. It is a member of the tetraspan family (spanning the membrane four times) found on platelets, B cell progenitors, activated lymphocytes, granulocytes, endothelial cells and epithelial cells. CD9 induces adhesion, platelet aggregation, and B cell development. CD9 has been shown to associate with CD63, CD81, CD82, and CD36 and to bind to β1 integrins. |
| Distribution |
| Platelets, B cell progenitors, activated lymphocytes, granulocytes, endothelial and epithelial cells. |
| View Products |
| Description |
| Interleukin 21 (IL-21) is a potent immunomodulatory cytokine mainly produced by NKT and CD4+ T-cells, particularly the inflammatory Th17 subset, and has pleiotropic effects on both innate and adaptive immune responses. These actions include positive effects such as enhancing proliferation of NK cells and cytotoxic T cells, and inhibitory effects on the antigen-presenting function of dendritic cells. It can also be proapoptotic for B cells and NK cells. Studies have shown that IL-21 is also an autocrine cytokine that potently induces Th17 differentiation, suppresses Foxp3 expression, and serves as a target for treating inflammatory diseases. |
| Distribution |
| Cellular Sources: NKT cells and T cells; Cellular Targets: Cytotoxic T cells (CTL) and Natural Killer (NK) cells that can destroy malignant or infected cells. |
| View Products |
| Description |
| Interleukin 21 receptor (IL-21R), is a single pass type I membrane protein and a member of the type I cytokine receptor family. Of the type I cytokine receptors, IL-21R exhibits greatest extracellular homology to the IL-2R beta subunit, i.e., contains one copy of the WSXWS-containing cytokine-binding domain. Intracellular domains of IL-21R include the Box 1 and Box 2 elements which are similar to the IL-9R intracellular region. Upon binding IL-21, the IL-21R forms a heterodimer with the common gamma subunit (CD132) and induces Jak/Stat signaling. IL-21R is expressed on B cells and at various levels on NK and T cells. IL-21 is a potent immunomodulatory cytokine mainly produced by NKT and CD4 T-cells (particularly the inflammatory Th17 subset) and has pleiotropic effects on both innate and adaptive immune responses. These actions include positive effects such as enhanced proliferation of natural killer (NK) cells and cytotoxic T cells that can destroy virally infected or cancerous cells and direct inhibitory effects on the antigen-presenting function of dendritic cells. It can also be proapoptotic for B cells and NK cells. Recent studies have shown that IL-21 is also an autocrine cytokine that potently induces Th17 differentiation and suppresses Foxp3 expression, and serves as a target for treating inflammatory diseases. |
| Distribution |
| Expressed on all mature lymphocytes including B, T and NK cells. Reported to be up-regulated upon activation and highest expression on follicular B cells. |
| View Products |
| Description |
| Interleukin 23 (IL-23) is a member of the IL-6 family of cytokines, and it is comprised of two subunits, p19 and p40. The p19/p40 heterodimer is stabilized by a disulfide bond. The p40 subunit is shared by IL-23 and IL-12 cytokines. p19 mRNA is expressed in endothelial cells and polarized T cells; p40 is not expressed by these cells. Therefore, the availability of functional IL-23 is limited by the expression of p40 and not p19. IL-23 exerts its biological activities through the interaction with a heterodimeric receptor complex composed of IL-12Rb1 and IL-23R. IL-23 activates Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling molecules. JAK2 is constitutively associated with the IL-23R chain, and binding of IL-23 to its receptor leads to phosphorylation of STAT1, STAT3, STAT4, and STAT5. IL-23 promotes Th17 responses in vivo, and it is important for the survival and population expansion of Th17 cells. IL-23 induces proliferation of memory T cells and the induction of IL-17A and IL-17F in these cells. In conjunction with IL-1, IL-23 is sufficient to induce naïve human T cells to produce IL-17A, IL-17F, IL-22, IL-26, IFN-γ, CCL20/MIP-3α, and the transcription factor RORγt. PGE2 favors the production of IL-23 and inhibit IL-12 in dendritic cells. PGE2 synergizes with IL-23 in expanding Th17 cells from purified memory CD4+ T cells activated in the absence of accessory cells. IL-23 induces IFN-gamma production from T cells treated with PHA. In addition, IL-23 plays a key role in the pathogenesis of autoimmune and chronic inflammatory disorders. This role is supported by the identification of IL-23 receptor (IL-23R) susceptibility alleles associated with inflammatory bowel disease, psoriasis, and ankylosing spondylitis. |
| Distribution |
| Cellular Sources: activated dendritic cells, macrophages, and epidermal Langerhans cells; Cellular Targets: Memory T cells, Th17 cells, Inflammatory macrophages, NK cells, dendritic cells, and monocytes. |
| View Products |
| Description |
| IL-23R associates with IL-12Rβ1 to form the receptor for IL-23. It mediates T cell, NK cell (and possibly macrophages/myeloid cell) stimulation. IL-23 has important functions in both innate and adaptive immunity. |
| Distribution |
| Monocytes, T cell subsets, NK cells, dendritic cells. |
| View Products |
| Description |
| IL-27 is a heterodimeric cytokine that consists of EBI3, an IL-12p40-related protein, and p28, an IL-12p35-related polypeptide. IL-27 can be allocated to the IL-6/IL-12 superfamily of cytokines which also includes IL-23. This family is defined based on similarities in the structural motifs of the ligands, such as a common four-helix bundle and their receptors, which contain a hematopoietin receptor domain. IL-27 signaling occurs via a receptor complex composed of the signal transducing receptor chains WSX-1 and glycoprotein (gp) 130. Whereas WSX-1 is the IL-27-specific receptor chain, gp130 is the common receptor subunit of IL-6 type cytokines. IL-27 is expressed at sites of inflammation in cytokine-driven autoimmune/inflammatory diseases, such as rheumatoid arthritis, psoriasis, inflammatory bowel disease, and sarcoidosis. |
| Distribution |
| Cellular Sources: Monocytes, macrophages, dendritic cells; Cellular Targets: Activated T cells, resting NK cells, resting NKT cells, neutrophils, eosinophils, mast cells, hepatic stellate cells, hepatoma cells, and hepatocytes. |
| View Products |
| Description |
| Also known as WSX-1, Cytokine receptor-like 1, and Type I T-cell cytokine receptor, IL-27Rα serves as the receptor for IL-27. It requires gp130 for signaling through STAT1 and STAT3. IL-27Rα promotes a Th1 response and is involved with innate immunity. |
| Distribution |
| T cells, NK cells, B cells, monocytes, intestinal epithelial cells, hepatocytes, neutrophils, mast cells, dendritic cells. |
| Description |
| IL-31, produced mainly by activated CD4+ T cells, is a newly discovered helical cytokine belonging to the gp130/IL6 cytokine family. IL-31 is closely related in structure to IL-6, IL-11, and IL-27. Binding of IL-31 to its receptor activates JAK/STAT, PI3K/AKT, and MAPK pathways. IL-31 acts on a broad range of immune- and non-immune cells and thus possesses potential pleiotropic physiological functions, such as regulating hematopoiesis and immune response. Human IL-31 is also involved in inflammatory bowel disease, airway hypersensitivity, and dermatitis. |
| Distribution |
| Cellular Sources: Low level in many tissues, activated CD4+ T cells, mast cells (human); Cellular Targets: Epithelial cells, keratinocytes, macrophages, monocytes. |
| View Products |
| Description |
| IL-31Rα pairs with OSMR to form the IL-31 receptor. IL-31Rα has previously been called GLMR (gp130-like monocyte receptor). IL-31Rα knockout mice display enhanced proliferation and expression of Th2 cytokines, but not Th1 cytokines. Therefore, IL-31Rα may limit Th2-related inflammation and immunity. |
| Distribution |
| Dendritic cells, keratinocytes, activated monocytes, macrophages, epithelial cells, neural cells. |
| Description |
| OSMR is also known as IL-31RB and Oncostatin M-specific receptor subunit beta. It is a type I cytokine receptor that heterodimerizes with IL-31Rα to signal for IL-31. It has been found to signal through STAT3 and possibly STAT1 and STAT5. OSMR deficiency has been associated with chronic skin itching. |
| Distribution |
| Neural cells, fibroblasts, epithelial cells, pre-B cells (mouse). |
| Description |
| Interleukin 32 (IL-32),previously known as a transcript (NK4), is produced by mitogen-activated lymphocytes, by IFNγ-activated epithelial cells or by IL-12 and IL-18-activated NK cells. Its expression is increased following activation of T-cells by mitogens or the activation of NK cells by IL-2.IL-32 activates NF-κ-B and p38 MAPK cytokine signal pathways. It has been suggested that IL-32 may play a role in autoimmune and inflammatory diseases such as rheumatoid arthritis.IL-32 is unusual in that it does not share sequence homology with known cytokine families and is highly expressed in immune tissues. IL-32 exists in at least four differentially spliced isoforms (α, β, γ and δ)with predicted molecular weight: ~26 kD.IL-32α is the shortest and most abundant of four potential splice variants of the pro-inflammatory cytokine IL-32.Potential modifications include myristoylation and N-glycosylation. Transfected IL-32 alpha was more likely to be cell-associated as compared to IL-32β, suggesting an intracellular function. |
| Distribution |
| Cellular Sources: Activated lymphocytes, keratinocytes, NK cells, fibroblast-like synoviocytes, endothelial cells; Cellular Targets: Monocytes, macrophages, dendritic cells, epithelial cells, osteoclasts. |
| View Products |
| Description |
| Human IL-34 shares a sequence identity of 99.6%, 72% and 71% with chimpanzee, rat, and mouse IL-34, respectively, on the amino acid level. The IL-34 gene is syntenic in the human, chimpanzee, rat, and mouse genomes. IL-34 has no apparent consensus structural domain or motif, and does not share sequence homology with M-CSF; nevertheless, it binds to the CSFR. These two cytokines are not identical in biological activity and signal activation. IL-34 and CSF show an equivalent ability to support cell growth or survival. However, these cytokines have differing ability to induce the production of chemokines (MCP-1 and eotaxin-2) in primary macrophages, the morphological change in TF-1-fms cells, and the migration of J774A.1 cells. The use of monoclonal antibodies against the CSFR suggests a differential domain binding in the receptor to IL-34 and CSF and, as a result, different bioactivities and signal activation kinetics/strength are produced for these cytokines. |
| Distribution |
| Cellular Sources: expressed in different tissues, including spleen, heart, brain, lung, liver, kidney, thymus, testes, ovary, small intestine, prostate, and colon; Cellular Targets: Monocytes, macrophages, osteoblasts, myeloid cells. |
| View Products |
| Description |
| CSF-1R, also known as CD115 and M-CSFR, is a single-pass type I membrane protein and member of the platelet-derived growth factor receptor family. Structural studies of CD115 have described an Ig-like extracellular domain, a transmembrane domain, an intracellular juxtamembrane domain, a split tyrosine kinase domain, and a C-terminal tail receptor. Receptor activation induces homo-dimerization in addition to phosphorylation and ubiquitinylation of intracellular residues. The natural ligands of CD115 include M-CSF and IL-34. CD115 directly influences tissue macrophage and osteoclast differentiation and proliferation. It is expressed on monocytes/macrophages, plasmacytoid and conventional dendritic cells, and osteoclasts. |
| Distribution |
| Monocytes/macrophages, plasmacytoid and conventional dendritic cells, osteoclasts, and peritoneal exudate cells (mouse). |
| View Products |
| Description |
| IL-35 is a newly described cytokine comprised of the IL-12α chain (p35) and the Epstein-Barr virus-induced gene product 3 (EBI-3). IL-35 belongs to the IL-12 family which includes IL-12, IL-23, IL-27, and IL-35. These cytokines are heterodimeric proteins comprised of an α chain (p19, p28, or p35) and a β chain (p40 or Ebi3). IL-35 suppresses T effector cell proliferation in vitro and induces a new subpopulation of Tregs (iTregs35). IL-35-Fc fusion protein enhances the proliferation of mouse Treg cells. Interaction between Tregs and T effector cells induces IL-35 production. IL-35 has recently been found to signal through a heterodimer of IL-12Rβ2 and gp130 or homodimers of each chain. |
| Distribution |
| Cellular Sources: Regulatory T cells (Treg), CD4+CD25+Foxp3+, CD4+CD25+, Foxp3- regulatory T cells; Cellular Targets: T conventional cells, T regs. |
| View Products |
| Description |
| IL-12Rβ2 is a protein receptor for interleukin-12. This subunit is the signaling component of the IL-12R complex, which utilizes the JAK2 and STAT4 pathway. It promotes the proliferation of T-cells and NK cells. It also biases T cells towards the Th1 phenotype by strongly enhancing IFN-gamma production. |
| Distribution |
| Activated T cells, naïve germinal center and memory B cells, and myeloma cells. |
| View Products |
| Description |
| IL-10 was first described as a cytokine that is produced by T helper 2 (Th2)cell clones. It inhibits interferon (IFN)-γ synthesis in Th1 cell, and therefore it was initially called cytokine synthesis inhibiting factor(CSIF). Macrophages are the main source of IL-10 and its secretion can be stimulated by endotoxin (via Toll-like receptor 4, NF-κB dependent), tumor necrosis factor TNF-α (via TNF receptor p55, NF-κB-dependent), catecholamines, and IL-1. IL-10 controls inflammatory processes by suppressing the expression of proinflammatory cytokines, chemokines, adhesion molecules, as well as antigen-presenting and costimulatory molecules in monocytes/macrophages, neutrophils, and T cells. IL-10 inhibits the production of proinflammatory mediators by monocytes and macrophages such as endotoxin- and IFN-γ-induced release of IL-1α, IL-6, IL-8, G-CSF, GM-CSF, and TNF-α. In addition, it enhances the production of anti-inflammatory mediators such as IL-1RA and soluble TNFα receptors. IL-10 inhibits the capacity of monocytes and macrophages to present antigen to T cells. This is realized by down-regulation of constitutive and IFNγ-induced cell surface levels of MHC class II, of costimulatory molecules such as CD86 and of some adhesion molecules such as CD58. |
| Distribution |
| Cellular Sources: Activated CD8+ and CD4+ T cells, activated monocytes, macrophages, keratinocytes, mast cells, Ly-1 B (mouse); Cellular Targets: T cells, B cells, mast cells, macrophages |
| View Products |
| Description |
| CD210, also known as the IL-10 receptor, is a protein expressed on T cells, B cells, NK cells, monocytes and macrophages. CD210 belongs to the class II cytokine receptor family which includes the IFN-γ receptor (CDw119), the IFN-α/β receptor (CD118) and tissue factor (CD142). The IL-10 receptor is involved in signal transduction by inducing phosphorylation of STAT1a and STAT3 and by inducing activation of Jak1 and Tyk. |
| Distribution |
| Thymocytes, T and B cells, NK cells, monocytes, macrophages. |
| View Products |
| Description |
| IL-10Rβ is also known as CDw210b. It is used as a cell surface receptor for several class 2 cytokines, including: IL-10, IL-22, Il-26, IL-28, and IL-29. Defects in this chain are associated with inflammatory bowel disease. |
| Distribution |
| Thymocytes, T and B cells, NK cells, monocytes, macrophages, neutrophils. |
| View Products |
| Description |
| A member of the IL-10 cytokine family, IL-19 is also known as melanoma differentiation associated protein-like protein. Treatment of monocytes with LPS or GM-CSF induces IL-19 production. IL-19 has been found to be elevated during endotoxic shock. It can also induce death in epithelial cells through reactive oxygen species and apoptosis. Recent data has shown IL-19 can promote Th2 development and anti-inflammatory responses. |
| Distribution |
| Cellular Sources: Synovial cells, monocytes, epithelial cells, dendritic cells, T cells, B cells; Cellular Targets: Keratinocytes, epidermal cells, endothelial cells, dendritic cells. |
| Description |
| IL-20 is a member of the IL-10 family of cytokines. IL-20 has been found to regulate the proliferation and differentiation of keratinocytes in an autocrine fashion. IL-20 is also involved with inflammatory diseases like psoriasis and rheumatoid arthritis. IL-20 increased colony formation and cell cycling of certain multipotential progenitor cells. It had no effect on erythroid, granulocyte-monocyte, or megakaryocyte progenitors. |
| Distribution |
| Cellular Sources: Keratinocytes, monocytes, epithelial cells; Cellular Targets: keratinocytes, endothelial cells, CD8+ T cells, certain multipotential progenitor cells. |
| Description |
| A member of the IL-10 cytokine family, IL-24 is also known as ST16 (suppressor of tumorigenicity-16) and MDA-7 (melanoma differentiation-associated gene 7). It was initially discovered in melanoma cells that lost the ability to differentiate and proliferate. It selectively suppressed the growth of a wide variety of cancer cells by apoptosis. |
| Distribution |
| Cellular Sources: Megakaryocytes, monocytes, macrophages, Th2 cells; Cellular Targets: Endothelial cells, wide range of cancerous cells. |
| Description |
| IL-20Rα is a protein paired with IL-20Rβ to serve as the receptor for IL-19, IL-20, and IL-24. It can also pair with IL-10Rβ to bind IL-26. |
| Distribution |
| Keratinocytes, epidermal cells; particularly expressed in cells of the skin, heart, brain, and testis. |
| Description |
| IL-20Rβ is also known as IL-20R2. It pairs with IL-20Rα to serve as a receptor for IL-19, IL-20, and IL-24. |
| Distribution |
| Keratinocytes, epidermal cells; particularly expressed in cells of the skin and testis. |
| Description |
| IL-22 is a cytokine structurally related to IL-10. Originally identified as a murine gene induced by IL-9 in T and mast cells, IL-22 was initially designated ILTIF (IL-10-related T cell-derived inducible factor). IL-22 belongs to a family of cytokines with limited homology to IL-10, namely IL-10, IL-19, IL-20, IL-24, IL-26, and the IFN-λs, IL-28A, IL-28B and IL-29. Human IL-22 shares 79% amino acid identity with murine IL-22 and 25% identity with human IL-10. IL-22 biological activity is initiated by binding to a cell surface complex composed of IL-22R1 and IL-10R2 receptor chains. Activity is further regulated through interactions with the soluble binding protein, IL-22BP, which shares sequence similarity with an extracellular region of IL-22R1 (sIL-22R1). Both chains of the IL-22R complex belong to the class II CRF. Two types of IL-22 binding receptors have been discovered, a membrane-bound receptor and a soluble receptor, both encoded by different genes. IL-22 is produced by Th17 cells and newly identified Th22 cells. It plays a critical role in mucosal immunity in addition to the deregulated inflammation observed in autoimmune diseases. |
| Distribution |
| Cellular Sources: thymic lymphomas, T cells, activated T cells, macrophages, and mast cells, Th17, lymphoid tissue induce (LTi) cells, subsets of natural killer cells; Cellular Targets: Epithelial cells, keratinocytes, hepatocytes, Th17 cells, pancreatic acinar cells. |
| View Products |
| Description |
| IL-22Rα1 is a component of the receptor for IL-20, IL-22, and IL-24. IL-22 signaling is conducted via the JAK/STAT, MAPK1/MAPK3, and Akt kinase pathways. Signaling for IL-20 and IL-24 are also conducted via the JAK/STAT pathways. IL-22Rα1 also mediates anti-angiogenic and endothelial cell inhibitory effects upon binding of IL-24. |
| Distribution |
| Keratinocytes, endothelial cells of the blood brain barrier. Cells of the central nervous system and synovial cells. Also found in liver, lung, and kidney tissue. |
| View Products |
| Description |
| IL-22Rα2 is also known as IL-22 binding protein, and has been found to prevent interaction of IL-22 with the functional complex consisting of IL-10Rβ and IL-22Rα1. This may help to limit the inflammatory response. |
| Distribution |
| Alveolar macrophages, monocytes, alveolar epithelial cells, neutrophils, dendritic cells. |
| Description |
| IL-26 , a member of the IL-10 cytokine family, was originally identified as the protein AK155. It was initially discovered in Herpesvirus Samirii-transformed T cells. Currently, there is no known mouse-counterpart. IL-26 binds to its receptor, which consists of IL-10Rβ and IL-20Rα, signaling through STAT1 and STAT3. It may play a role in mucosal immunity. IL-26 also has a pro-inflammatory function and may be involved with inflammatory bowel disease. |
| Distribution |
| Cellular Sources: memory T cells, Th17 cells, TH2 cells, NK cells; Cellular Targets: Epithelial cells, keratinocytes, hepatocytes. |
| Description |
| Interleukin-28 (IL-28, IFN-λ2, IFN- λ3) is a class II cytokine receptor ligand distantly related to type I interferons (IFNs) and the IL-10 family. IL-28 has two isoforms,IL-28A (IFN-λ2) and IL-28B (IFN-λ3) that are similar in amino acid sequence to IL-29 (IFN-λ1), as IL-29 shows 81% homology with IL-28A. IL-28 and IL-29 are produced by human peripheral blood mononuclear cells and dendritic cells upon viral infection, LPS stimulation, TLR stimulation, stimulation with poly(I:C) and other inducers of type I interferons. IL-28 and IL-29 both play a role in the interferon-mediated immune defense against viruses and up-regulate MHC class I antigen expression. |
| Distribution |
| Cellular Sources: wide variety of nucleated cell types, particularly dendritic cells; Cellular Targets: Epithelial cells, hepatocytes. |
| View Products |
| Description |
| Interleukin-28 (IL-28, IFN-λ2, IFN- λ3) is a class II cytokine receptor ligand distantly related to type I interferons (IFNs) and the IL-10 family. IL-28 has two isoforms,IL-28A (IFN-λ2) and IL-28B (IFN-λ3) that are similar in amino acid sequence to IL-29 (IFN-λ1), as IL-29 shows 81% homology with IL-28A. IL-28 and IL-29 are produced by human peripheral blood mononuclear cells and dendritic cells upon viral infection, LPS stimulation, TLR stimulation, stimulation with poly(I:C) and other inducers of type I interferons. IL-28 and IL-29 both play a role in the interferon-mediated immune defense against viruses and up-regulate MHC class I antigen expression. |
| Distribution |
| Cellular Sources: mononucleated cell types, particularly dendritic cells; Cellular Targets: Epithelial cells, hepatocytes. |
| Description |
| Interleukin-29 (IL-29, IFN-λ1) is a class II cytokine receptor ligand distantly related to type I interferons (IFNs) and the IL-10 family. IL-29 is similar in amino acid sequence to IL-28A (IFN-λ2) and IL-28B (IFN-λ3) as IL-29 shows 81% homology with IL-28A. IL-28 and IL-29 are produced by human peripheral blood mononuclear cells and dendritic cells upon viral infection, LPS stimulation, TLR stimulation, stimulation with poly(I:C) and other inducers of type I IFNs. IL-28 and IL-29 play a role in the IFN-mediated immune defense against viruses and up-regulates MHC class I antigen expression. |
| Distribution |
| Cellular Sources: mononuclear cells, particularly dendritic cells, monocytes; Cellular Targets: Keratinocytes, macrophages , dendritic cells, epithelial cells, hepatocytes. |
| View Products |
| Description |
| The IL-28RA, also known as IFNLR1, CRF-2-12, and LICR2, is a 57kD single-pass type I membrane glycoprotein containing one fibronectin III domain. Widely expressed in many tissue types, IL-28RA and IL-10RB dimerize to form the class II cytokine receptor for IL-28A (IFNλ2), IL-28B (IFNλ1) and IL-29 (IFNλ3), also known as the type III interferons. Like type I interferons, the type III interferons have been shown to activate the Jak-STAT and MAPK signaling pathways and exhibit antiviral activity. |
| Distribution |
| Broad tissue distribution at low level. |
| View Products |
ProductsHere






Follow Us