IBEX: A Highly Multiplexed Antibody-Based Application for 65+ Color Microscopy
Highly multiplexed single-cell technologies such as spectral flow cytometry and new methodologies like single-cell multiomics have greatly expanded the ability to simultaneously analyze multiple parameters on a single cell. While single-cell multiomic technologies provide a wealth of data related to cell state and phenotypes, none of these techniques offer insight into the tissue superstructure or spatial relationships of cells within entire organs. These relationships are critical for understanding the biology of cells and proteins as it relates to in vivo function and pathogenesis. This is where investigators turn to conventional techniques like colorimetric or fluorescent immunohistochemistry. However, there is a limit to the number of parameters that can be simultaneously analyzed with these conventional methods, as they are typically limited by the number of the unique chromogens or fluorophores that can be deconvoluted in a single imaging cycle.
Highly multiplexed microscopy methods now allow for the analysis of dozens of parameters (up to 50 or more) within a single tissue section. These methods may utilize different technologies to achieve simultaneous detection of dozens of parameters. These include mass spectrometry-based detection, DNA-oligo conjugated antibodies with fluorescent DNA probes, and cyclical techniques where common fluorophore-conjugated antibodies have their signal masked or destroyed to allow for multiple staining/imaging cycles (1).
If you’re interested in the IBEX technology and how BioLegend’s antibodies work in this application, contact our field application scientist team.
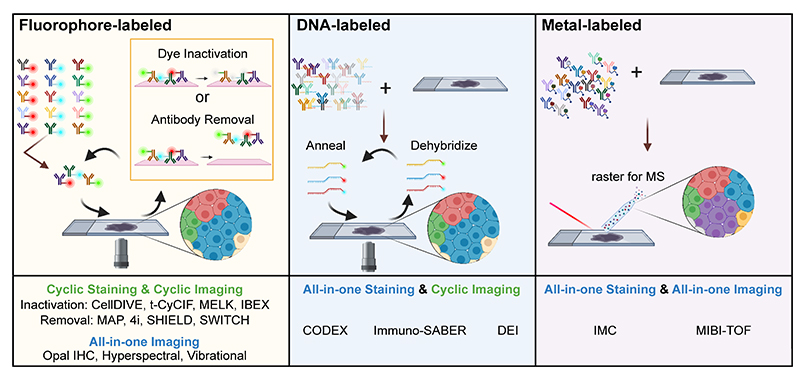
Figure 1. Cartoon depicting several types of multiplexed antibody-based imaging methods as depicted in the recent antibody-based imaging review (3). Image credit: John Hickey.
Iterative Bleaching Extended multi-pleXity (IBEX) Method
Dr. Andrea Radtke and colleagues of the Lymphocyte Biology Section in the National Institute of Allergy and Infectious Diseases (NIAID, NIH) have recently developed an open cyclical imaging technique, Iterative Bleaching Extended multi-pleXity (IBEX), that utilizes conventional microscopes and requires only basic laboratory skills to implement. IBEX allows for repeated cycles of staining, imaging, and bleaching, conveniently using hundreds of commercially available antibodies. Using IBEX, the laboratory of Dr. Ronald Germain has demonstrated high resolution imaging in a wide range of mouse and human tissues, including lymph nodes, spleen, thymus, liver, kidney, small intestine, skin, and lung. The IBEX method can be implemented at various scales and is not restricted to specific instrumentation. Below is a representative image of a human mesenteric lymph node acquired using the IBEX method and over 60 commercially available antibodies targeting various protein biomarkers in the tissue (2, 3).
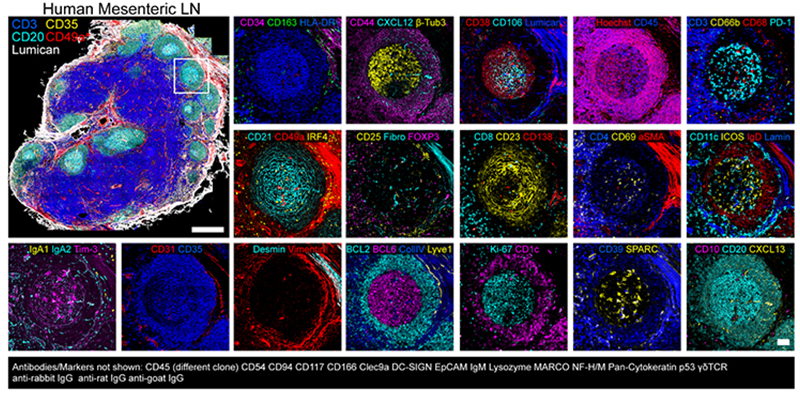
Figure 2. Spatial immunoprofiling of human mesenteric lymph node using IBEX (3).
How IBEX Works
IBEX is an open and flexible multiplex imaging method that can be implemented by most laboratories. Central to the technique is the use of the bleaching agent, lithium borohydride (LiBH4), to inactivate the fluorescent signal from a wide range of fluorescently conjugated antibodies.
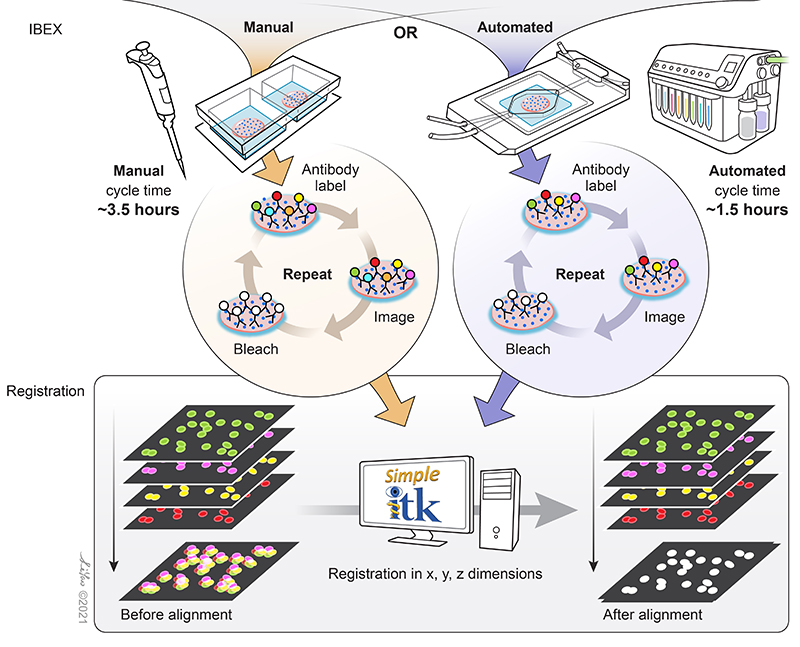
Figure 3. Schematic of the simple principles that allow IBEX to enable high-content imaging. Image credit: Graphical artist Li Yao.
Fluorophore inactivation with borohydride containing compounds has been previously described (4), and LiBH4 bleaching has been shown to be compatible with many familiar commercial fluorophore conjugates such as Alexa Fluor® dyes, Brilliant Violet™ dyes, FITC, PE, and more. Thus far, over 100 unique BioLegend reagents have been tested and used to generate high-parameter images using IBEX (2, 3) in a wide range of human and mouse tissues. Different dyes provide versatility in their use for the technique. Some bleach rapidly, allowing for quick removal of signal originating from specific targets. Others, such as Alexa Fluor 594®, are more resilient to LiBH4 bleaching, allowing for specific markers to be preserved across multiple imaging cycles. This permits antibodies against specific targets to be used across staining cycle panels and aid in image registration.
The image registration software developed to align IBEX-generated images employs open source software that can be operated without programming experience. Extensive documentation on the Simple ITK software developed by Dr. Ziv Yaniv and Bradley Lowekamp of the Bioinformatics and Computational Bioscience Branch of NIAID, NIH are listed here along with its full instructions (also read the README.md file which is part of the zip file). Additional details can be found in the XT Register Same Channel Simple ITK Imaris Python Extension YouTube tutorial. Sample data can be accessed on Zenodo for usage of the software.
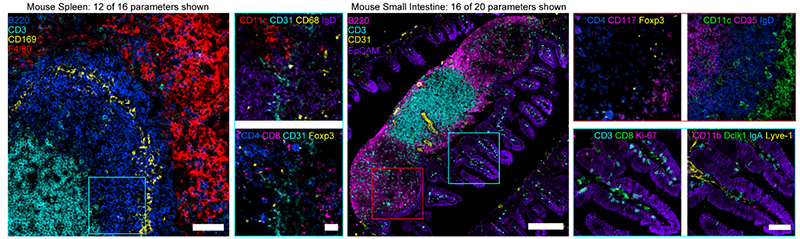
Figure 4. (Left) Confocal images from mouse spleen (12 of 16 parameters shown). Scale bar is 50 µm (left), 20 µm (insets). (Right) Confocal images from mouse small intestine (16 of 20 parameters shown). Scale bar is 200 µm (left), 25 µm (insets). Figures adopted from Radtke et al., 2020 (2).
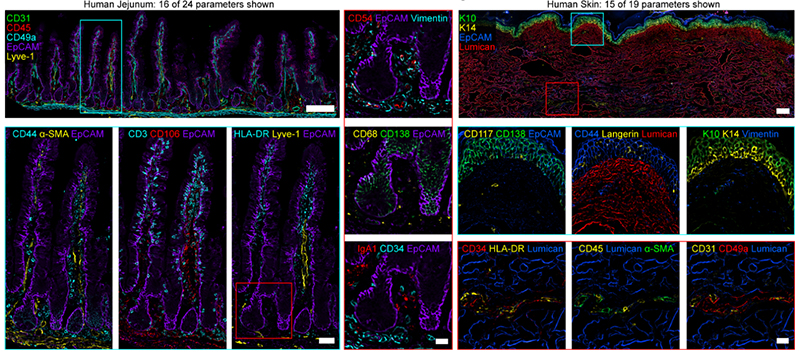
Figure 5. (Left) Widefield images from human jejunum (16 of 24 parameters shown). Scale bar is 200 µm (left), 50 µm (cyan box), 25 µm (red box). (Right) Widefield mages from human skin (15 of 19 parameters shown). Scale bar is 200 µm (left), 25 µm (insets). Keratin 10 (K10), Keratin 14 (K14). Figures adopted from Radtke et al., 2021 (1).
Radtke et al. extensively describe the considerations around implementation of IBEX for both manual and automated workflows in a recent protocol publication: IBEX: An open and extensible method for high content multiplex imaging of diverse tissues (2).
To learn more about the types of discoveries being unlocked using IBEX, view the recent presentation of findings using IBEX.
Highly multiplexed imaging techniques such as IBEX will continue to evolve and potentially incorporate multiple data modalities (RNA, DNA, etc.) or other applications such as thick or whole organ imaging using Ce3D™ Tissue Clearing Solution, a tissue clearing technology developed in the laboratory of Dr. Ronald Germain (5). As such, the insights gained to spatial biology and disease will be invaluable, particularly in the areas of systems biology and cancer research. In the coming months, BioLegend will be featuring IBEX as a reported technique and will expand on both compatible reagents and future directions of the technology.
Contributed by Josh Croteau, Ph.D., BioLegend.

Artwork was created by Autumn Yarmosh and David M. Sullivan.
References:
- Radtke, Andrea J et al. “IBEX: A versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues.” Proceedings of the National Academy of Sciences of the United States of America vol. 117,52 (2020): 33455-33465. doi:10.1073/pnas.2018488117
- Radtke, Andrea J et al. “IBEX: an iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues.” Nature Protocols vol. 17,2 (2022): 378-401. doi:10.1038/s41596-021-00644-9
- Hickey, John W et al. “Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging.” Nature Methods vol. 19,3 (2022): 284-295. doi:10.1038/s41592-021-01316-y
- Radtke Andrea J., et al. (2021). Accompanying dataset for: "IBEX: An iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues" [Data set]. Zenodo. doi.org/10.5281/zenodo.5244551
- Du, Z., Lin, JR., Rashid, R. et al. Qualifying antibodies for image-based immune profiling and multiplexed tissue imaging. Nat Protocols 14, 2900–2930 (2019). doi.org/10.1038/s41596-019-0206-y
- Radtke, A. et al. “A multi-scale, multiomic atlas of human normal and follicular lymphoma lymph nodes”. bioRxiv 2022.06.03.494716; doi.org/10.1101/2022.06.03.494716
Release:
The topics discussed herein are not an endorsement of BioLegend or its products by the National Institute of Allergy and Infectious Diseases or National Institutes of Health.






Follow Us