- Other Names
- Tumor necrosis factor-α, Cachectin, Necrosin, Macrophage cytotoxic factor (MCF), Differentiation inducing factor (DIF), TNFSF2
- Ave. Rating
- Submit a Review
- Product Citations
- publications
-

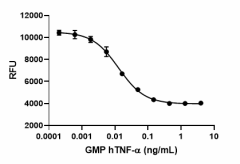
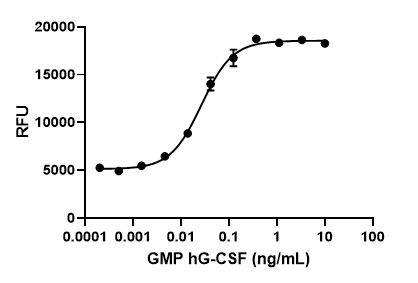
GMP recombinant human TNF-α induces cytotoxicity of L929 cells in a dose-dependent manner with an ED50 range of 0.01 - 0.085 ng/mL.
| Cat # | Size | Price | Quantity Check Availability | Save | ||
|---|---|---|---|---|---|---|
| 570114 | 25 µg | 254€ | ||||
| 570116 | 100 µg | 756€ | ||||
TNF-α was originally described as an endotoxin-induced, macrophage-derived factor that promotes hemorrhagic necrosis of solid tumors and the cachexia of chronic infections. TNF-α has also been implicated in a range of inflammatory, infectious, and malignant disorders. At the cellular level, TNF-α modulates a broad spectrum of responses including inflammation, immunoregulation, proliferation, apoptosis, and antiviral activity. In bone, the cytokine inhibits extracellular matrix deposition, stimulates matrix metalloprotease synthesis, and enhances production of osteoclastogenic cytokines such as M-CSF and RANKL. Chronic exposure to TNF-α in vivo increases osteoclastogenesis through two distinct mechanisms. TNF-α first affects osteoclastogenesis at the osteoclast precursor stage in the bone marrow by priming these cells to differentiate into cFms+/CD11b+/RANK+/- osteoclast progenitors via a RANKL/RANK independent mechanism. These osteoclast precursors then enter the blood and peripheral tissues where they differentiate into mature osteoclasts in the presence of RANKL, and this process is accelerated by TNF. The role of TNF at this later stage of osteoclast differentiation is RANKL/RANK dependent. Importantly, TNF-α promotes bone resorption both in vitro and in vivo by enhancing the proliferation and differentiation of osteoclast precursors.
Product DetailsProduct Details
- Source
- Human TNF-α, amino acids Val77-Leu233 (Accession# NM_000594), was expressed in E. coli.
- Molecular Mass
- The 157 amino acid recombinant protein has a predicted molecular mass of 17.35 kD. The DTT-reduced protein and non-reduced protein migrate at approximately 16 kD by SDS-PAGE. The N-terminal amino acid is Val.
- Purity
- > 95%, as determined by Coomassie stained SDS-PAGE
- Formulation
- 0.1 µm filtered protein solution is in 10 mM NaH2PO4, 150 mM NaCl, pH 7.2.
- Endotoxin Level
- Less than 0.1 EU per µg protein as determined by the LAL method
- Concentration
- 500 µg/mL
- Storage & Handling
- Unopened vial can be stored between 2°C and 8°C for up to 2 weeks, at -20°C for up to six months, or at -70°C or colder until the expiration date. For maximum results, quick spin vial prior to opening. The protein can be aliquoted and stored at -20°C or colder. Stock solutions can also be prepared at 50 - 100 µg/mL in appropriate sterile buffer, carrier protein such as 0.2 - 1% endotoxin-free BSA or HSA can be added when preparing the stock solution. Aliquots can be stored between 2°C and 8°C for up to one week or stored at -20°C or colder for up to 3 months. Avoid repeated freeze/thaw cycles.
- Activity
- ED50 = 0.01 - 0.085 ng/mL as determined by the dose-dependent cytotoxicity of L929 cells stimulated with actinomycin D.
- Application
-
Bioassay
Cell Culture - Application Notes
-
BioLegend carrier-free recombinant proteins provided in liquid format are shipped on blue-ice. Our comparison testing data indicates that when handled and stored as recommended, the liquid format has equal stability and shelf-life compared to commercially available lyophilized proteins after reconstitution. Our liquid proteins are verified in-house to maintain activity after shipping on blue ice and are backed by our 100% satisfaction guarantee. If you have any concerns, contact us at tech@biolegend.com.
- Disclaimer
-
GMP Recombinant Proteins. BioLegend GMP recombinant proteins are manufactured in a dedicated GMP facility and compliant with ISO 13485:2016. For research or ex vivo cell processing use. Not for use in diagnostic or therapeutic procedures. Our processes include:
- Batch-to-batch consistency
- Material traceability
- Documented procedures
- Documented employee training
- Equipment maintenance and monitoring records
- Lot-specific certificates of analysis
- Quality audits per ISO 13485:2016
- QA review of releaed products
BioLegend GMP recombinant proteins are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12.
Antigen Details
- Structure
- TNF superfamily
- Distribution
-
It is produced primarily by macrophages, and it is also expressed by activated T cells, B cells, NK cells, and neutrophils.
- Function
- Type II integral membrane protein processed by TACE for secretion; upregulated by interferons, IL-2, GM-CSF, substance P, bradykinin, PAF, immune complexes, cyclooxygenase; downregulated by IL-6, TGF-β, vitamin D3, prostaglandin E2, PAF antagonists
- Interaction
- Monocytes, neutrophils, macrophages, T cells, fibroblasts, endothelial cells, osteoclasts, adipocytes, astroglia, microglia
- Ligand/Receptor
- TNFRSF1A (TNF-R1, CD120a, TNFR-p60 Type β, p55); TNFRSF1B (TNF-R2, CD120b, TNFR-p80 Type A, p75)
- Bioactivity
- Measured by its ability to induce cytotoxicity of L929 cells stimulated with actinomycin D
- Biology Area
- Cell Biology, Immunology, Innate Immunity, Neuroinflammation, Neuroscience
- Molecular Family
- Cytokines/Chemokines
- Antigen References
-
- Boyce BF, et al. 2005. J Med 54:127-131.
- Lam J, et al. 2000. J Clin Invest 106:1481-1488.
- Udagawa N, et al. 2002. Arthritis Res 4:281-289.
- Kwon J, et al. 1993. Gene 132:227-236.
- Harth S, et al. 2019. MAbs. 11:178.
- de Oliveira Mann CC, et al. 2019. Cell Rep. 27:1165.
- Anderson NR, et al. 2019. Cell Adh Migr. 13:163.
- Zhichao Fan, et al. 2019. Cell Rep. 26(1):119-130.
- VanDussen KL, et al. 2019. Stem Cell Res. 37:101430.
- Hurrell BP, et al. 2019. Cell Rep. 29:4509.
- Gene ID
- 7124 View all products for this Gene ID
- UniProt
- View information about TNF-alpha on UniProt.org
 Login / Register
Login / Register 















Follow Us