The Importance of Quantitative Immunoassays to Measure Antibodies in Response to SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the infectious agent responsible for the worldwide Coronavirus Disease 2019 (COVID-19) pandemic1. It has become imperative that health officials and researchers have access to assay options that can help generate surveillance data to inform policy and implementation. In this blog, we describe how highly sensitive quantitative ELISAs for SARS-CoV-2 antibodies will help us better understand the nature and duration of immunity against SARS-CoV-2.
A
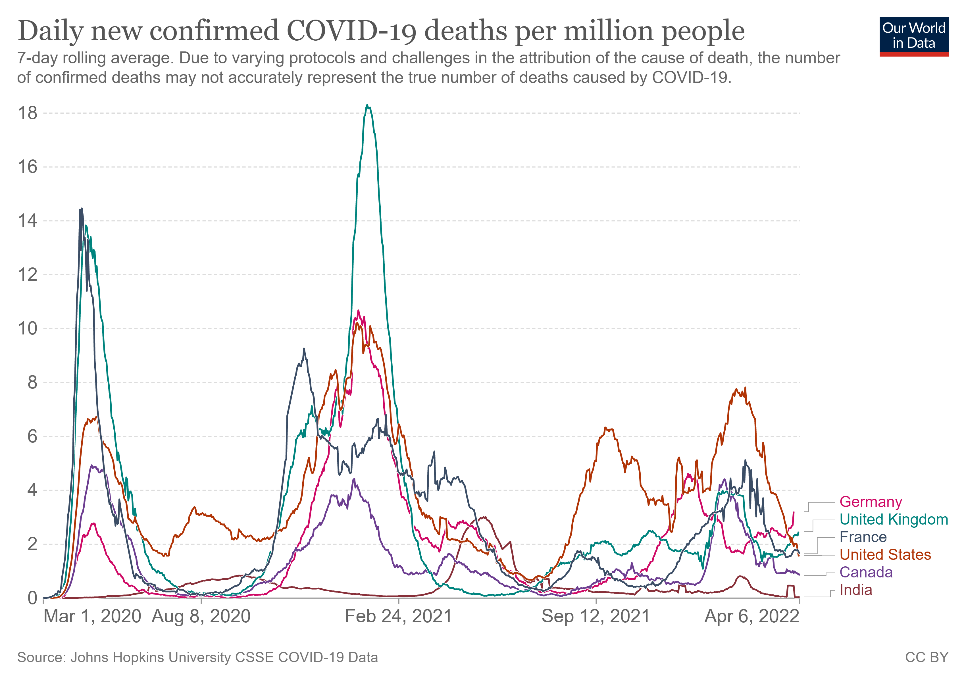
B
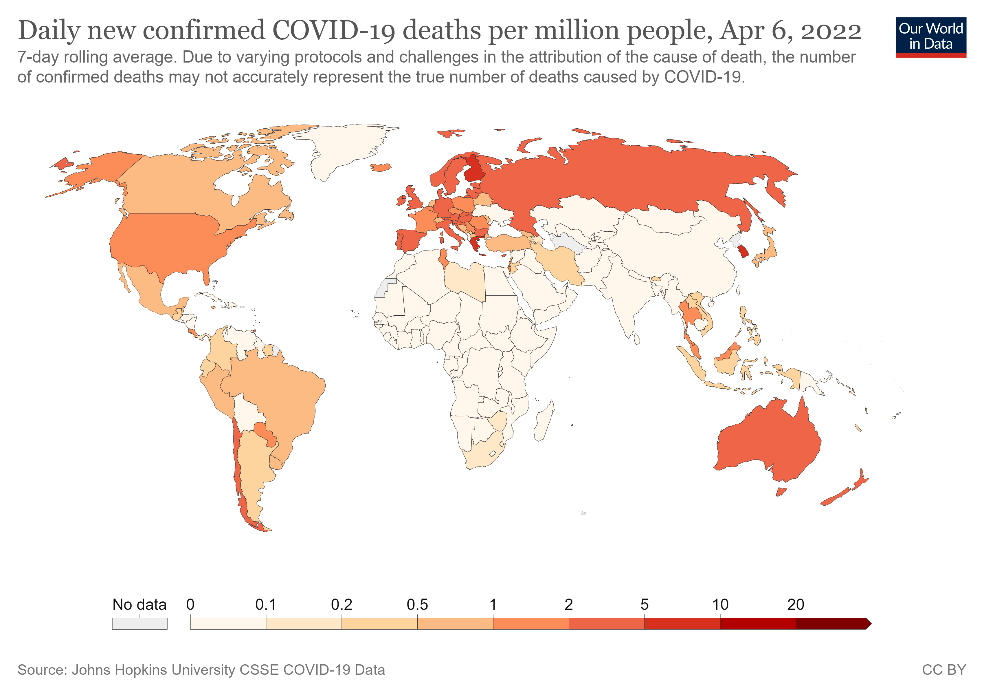
Figure 1. Confirmed COVID-19 deaths per million people from March 2020 to April 2022 in selected countries (A) and globally (B).
As of date, SARS-CoV-2 has led to more than 218 million confirmed cases and resulted in over 4.5 million deaths (Figs 1A-B)2. Since 2019, the global population has been in various forms of confinement to help limit viral transmission, and such restrictions have come with great socioeconomic costs3.
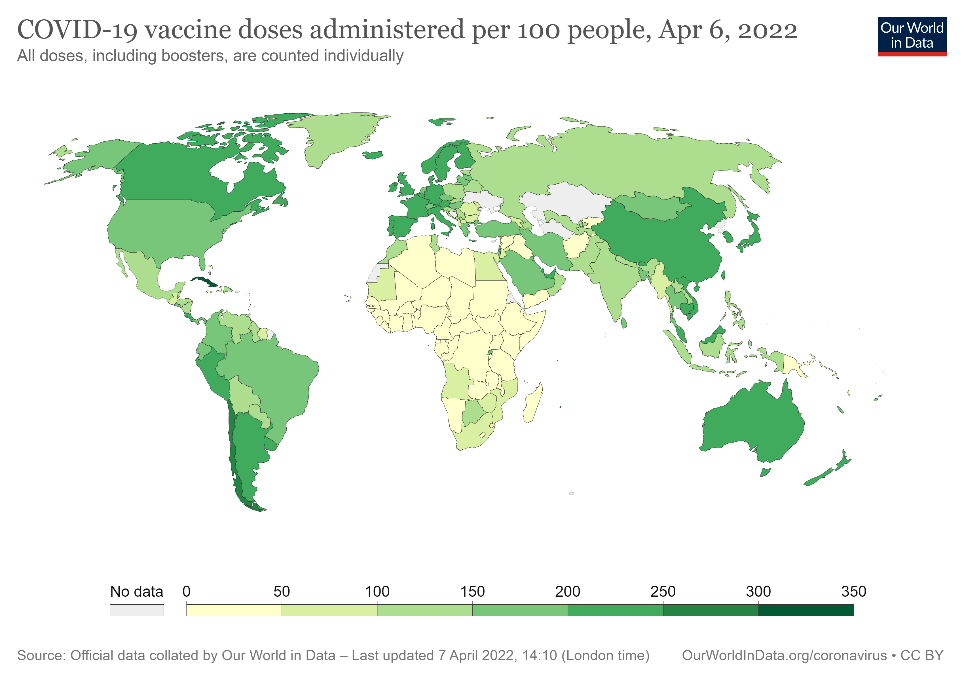
Figure 2. COVID-19 vaccine doses per 100 people administered globally from December 2020 to April 2022.
In order to move toward recovery, more than 5.3 billion doses of COVID-19 vaccines have been administered worldwide, with 371 million in USA since December 2020 (Fig 2)2. While those numbers are encouraging, it is estimated that even with the latest global vaccination rate of 41.4 million doses per day, a high level of global immunity remains a long way off (Fig 2). Given the current situation, and the possibility that SARS-CoV-2 may come to routinely circulate among populations as the common cold does, it is vital that researchers and health officials have a number of assay options. Of these, antibody testing (sometimes referred to as serological testing) is poised for prominence as it offers insight into durability and duration of vaccine-induced immunity and protection. Of utmost importance will be the ability to measure the effectiveness of an antibody response to SARS-CoV-2 antigens during a natural infection or to antigens presented in a vaccine.
General Biology of SARS-CoV-2 Virus
SARS-CoV-2 is a novel positive-sense, single-stranded enveloped RNA virus belonging to the Coronaviridae family (Fig 3). Its genome is about 30 kb in size and, once inside host cells, encodes spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. The lipid envelope (E) contains proteins involved in the production and maturation of virions. The membrane or matrix (M) glycoprotein spans the viral envelope and can bind to all the other structural proteins, thereby playing an important role in determining the shape of the virus envelope. By binding to the nucleocapsid (N) protein, the M protein also helps stabilize the N protein-RNA complex and facilitates the morphogenesis phase of the viral replication cycle4.
The spike (S) glycoprotein is a trimeric transmembrane protein that studs the surface of virions and facilitates attachment and fusion of the virus to host cell membrane and eventual entry into host cells. Each S protein monomer is divided into two functional subunits, S1 and S2. The S1 subunit contains a receptor-binding domain (RBD) that binds to the angiotensin-converting enzyme 2 (ACE2) receptor to enter host cells, while the S2 subunit facilitates membrane fusion as part of viral entry into cells4.
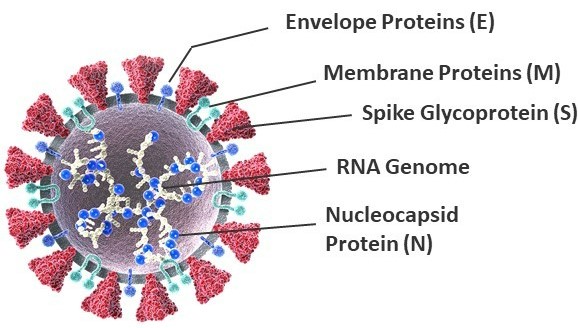
Figure 3. Molecular structure of the SARS-CoV-2 virus.
Immune Response to SARS-CoV-2 and the Need for Highly Sensitive, Quantitative ELISAs
Given the spike protein is large, exposed, and plays a direct role in mediating viral entry into host cells, it is the primary target of current COVID-19 vaccines5. Following vaccination or infection, the host immune system generates both cellular and humoral responses to SARS-CoV-2 antigens. However, the effectiveness of any vaccine depends on the robustness of the antibody response to targeted antigens to neutralize the virus life cycle. Evidence compiled shows that SARS-CoV-2 elicits a classic viral response from IgG, IgM, and IgA antibodies, which remain in circulation for months6.
As we move forward, testing antibody levels is even more important. Qualitative tests are often the first-line tools for general COVID-19 testing and inform whether or not antibodies are present in a patient’s blood. However, such a test only tells us that the patient has developed an immune response. Quantitative tests, such as ELISAs, are needed to tell us the robustness of the immune response. These assays are needed to reveal the temporal immunological profiles of SARS-CoV-2 infection and vaccine effectiveness. Quantitative ELISAs can tell us the prevalence of SARS-CoV-2 virus in a population, the durability of the protection provided by COVID-19 vaccines or natural infection, and our immune response to emerging variants, to name just a few priority research areas.
The majority of vaccines now in use target SARS-CoV-2 spike (S) protein, as that antigen is considered the keystone for optimal immunization and generation of neutralizing IgG antibodies. Learn how our LEGEND MAX™ SARS-CoV-2 Spike S1 Human IgG (Cat. No. 447807) and LEGEND MAX™ SARS-CoV-2 Spike RBD Human IgG (Cat. No. 447707) kits precisely quantitate SARS-CoV-2 spike-specific humoral response.
LEGEND MAX™ SARS-CoV-2 Spike Protein S1 IgG ELISA Kit
LEGEND MAX™ SARS-CoV-2 Spike Protein RBD IgG ELISA Kit
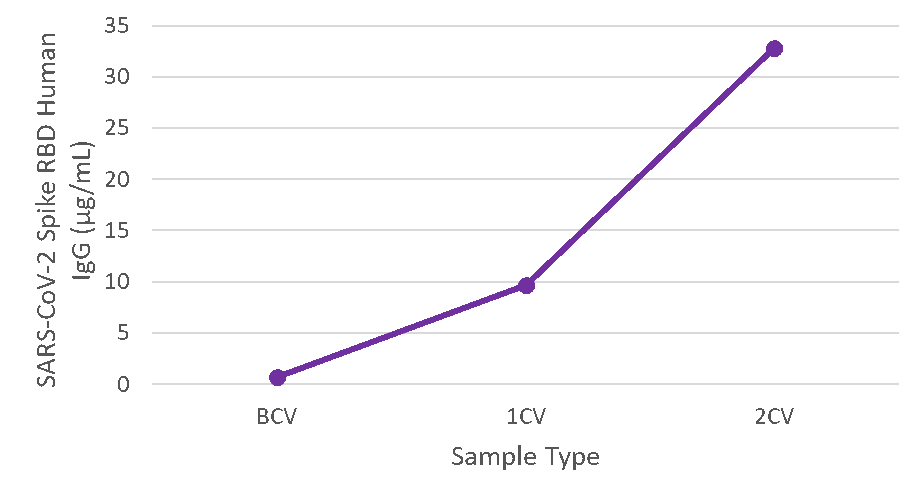
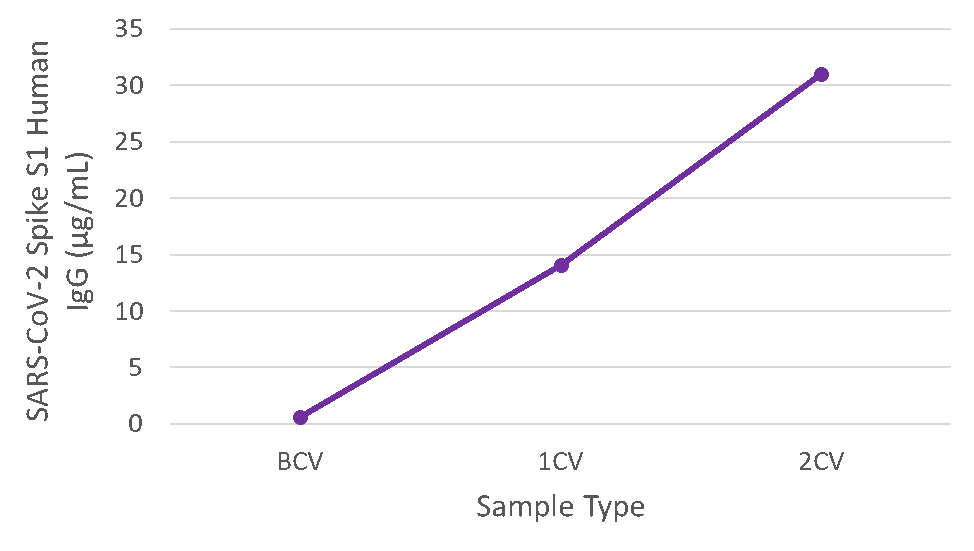
Figure 4. Serum samples were taken before vaccination (BCV), 2 weeks after first dose (1CV), and 2 weeks after second dose (2CV) of the Moderna or Pfizer SARS-CoV-2 vaccines, then tested for IgG concentration (n=26).
For even faster survey results, our new RAPID MAX™ SARS-CoV-2 Spike S1 Human IgG and RAPID MAX™ SARS-CoV-2 Spike RBD Human IgG kits yield highly sensitive, quantitative data on SARS-CoV-2 antibodies in less than 90 mins.
RAPID MAX™ SARS-CoV-2 Spike Protein S1 IgG ELISA Kit
RAPID MAX™ SARS-CoV-2 Spike Protein RBD IgG ELISA Kit
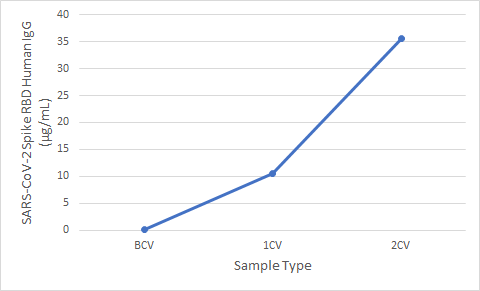
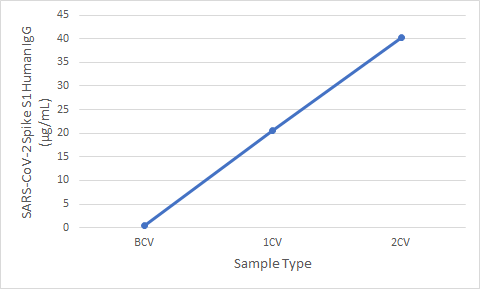
Figure 5. Serum samples were taken before vaccination (BCV), 2 weeks after first dose (1CV), and 2 weeks after second dose (2CV) of the Moderna or Pfizer SARS-CoV-2 vaccines, then tested for IgG concentration (n=26).
Reports have suggested different immunoglobulin types are produced in response to SARS-CoV-26. Our LEGEND MAX™ SARS-CoV-2 Spike RBD Human IgM (Cat. No. 448307) and LEGEND MAX™ SARS-CoV-2 Spike S1 Human IgM (Cat. No. 448207) offer quantitative measures of IgM antibodies for a more complete profile of the timing, duration, and effectiveness of humoral immune responses against SARS-CoV-2 infection or vaccination.
LEGEND MAX™ SARS-CoV-2 Spike Protein S1 IgM ELISA Kit
LEGEND MAX™ SARS-CoV-2 Spike Protein RBD IgM ELISA Kit
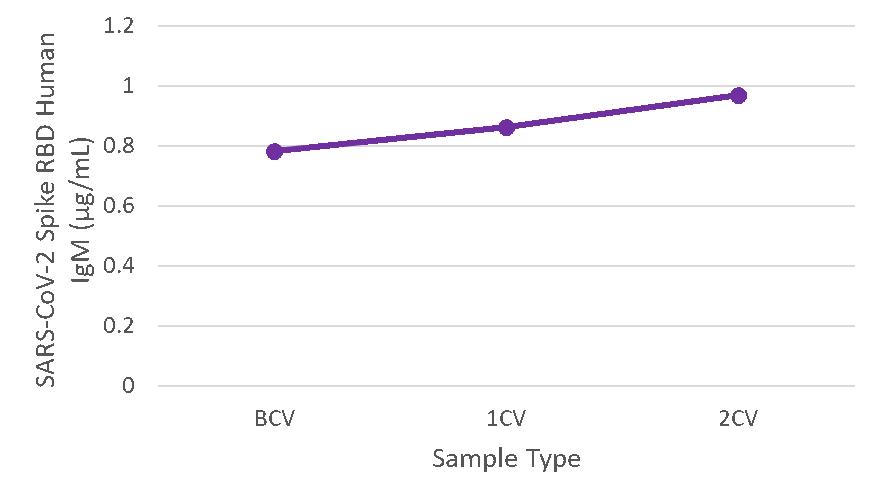
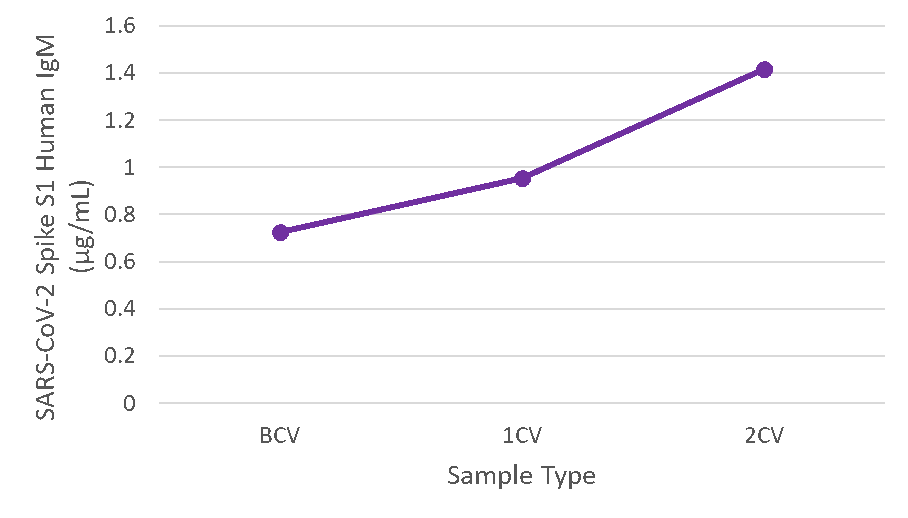
Figure 6. Serum samples were taken before vaccination (BCV), 2 weeks after first dose (1CV), and 2 weeks after second dose (2CV) of the Moderna or Pfizer SARS-CoV-2 vaccines, then tested for IgM concentration (n=26).
Antibodies to the nucleocapsid proteins (N) also serve as a valuable serological marker to infection or vaccination, and could be used as a measure of past infection as well as severity of the COVID-19 disease6. However, the role of these anti-N antibodies in neutralizing SARS-CoV-2 still needs to be established. Our LEGEND MAX™ SARS-CoV-2 Nucleocapsid Human IgG (Cat. No. 448107) will provide quantification of anti-N antibody to help resolve these important research questions.
The aforementioned ELISAs are instrumental in measuring antibodies generated against specific SARS-CoV-2 protein, but separate ELISAs would need to be conducted to measure antibodies against each of them. To save on workflow and samples, LEGENDplex™ immunoassays utilize multiple beads, with each bead conjugated to a specific immobilized SARS-CoV-2 protein on its surface that serves to capture a target antibody. Each population of beads is distinguished by size and internal fluorescence, allowing for the simultaneous analysis of antibodies generated to multiple SARS-CoV-2 proteins in a single sample. Learn more how our newest multiplex assays offer reliable measures of circulating human IgG, IgM, and IgA antibodies in response to SARS-CoV-2 infection and/or vaccination.
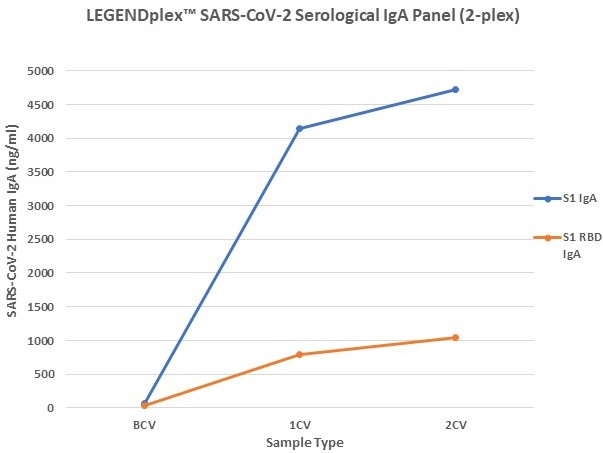

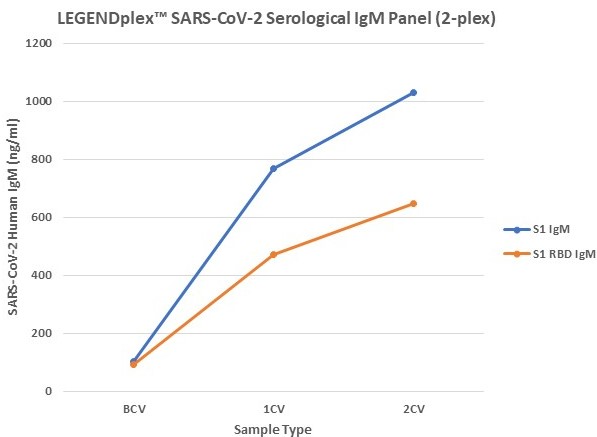
Figure 7. Serum samples were taking before vaccination (BCV), 2 weeks after first dose (1CV), and 2 weeks after second dose (2CV) of the Moderna or Pfizer SARS-CoV-2 vaccines, then tested for IgA, IgG, and IgM concentration (n=32).
To get a strong hold on COVID-19, it is imperative to understand all stages of infection: how SARS-CoV-2 recognizes and attacks host cells, how our immune system responds to that attack, and the pathogenesis or recovery post-infection. BioLegend offers exceptional tools to researchers combating SARS-CoV-2 at all of these stages. In this blog, we emphasize the critical need for highly sensitive, quantitative ELISAs to measure antibodies raised against SARS-CoV-2 spike-specific or nucleocapsid proteins. It is with quantified serological measures that we can move toward a stage in which we know in a given population how much protective immunity is circulating, the longevity/durability of the humoral immune responses, and efficacy of vaccines. We are dedicated to supporting top-quality research on SARS-CoV-2 so the global population can put COVID-19 behind us.
Resources:
- Wu, Fan et al. “A new coronavirus associated with human respiratory disease in China.” Nature vol. 579,7798 (2020): 265-269. doi:10.1038/s41586-020-2008-3. PubMed
- Ritchie, Hannah, et al. “Coronavirus Pandemic (COVID-19).” Our World in Data, 5 Mar. 2020, https://ourworldindata.org/coronavirus.
- Yeyati, Eduardo Levy, and Federico Filippini. “Social and Economic Impact of Covid-19.” Brookings, Brookings, 9 Mar. 2022, http://www.brookings.edu/research/social-and-economic-impact-of-covid-19/.
- Alsobaie, Sarah. “Understanding the Molecular Biology of SARS-CoV-2 and the COVID-19 Pandemic: A Review.” Infection and drug resistance vol. 14 2259-2268. 16 Jun. 2021, doi:10.2147/IDR.S306441. PubMed
- Dai, Lianpan, and George F Gao. “Viral targets for vaccines against COVID-19.” Nature reviews. Immunology vol. 21,2 (2021): 73-82. doi:10.1038/s41577-020-00480-0. PubMed
- Khoshkam, Zahra et al. “Recovery scenario and immunity in COVID-19 disease: A new strategy to predict the potential of reinfection.” Journal of advanced research vol. 31 (2021): 49-60. doi:10.1016/j.jare.2020.12.013. PubMed
 Login / Register
Login / Register 






Follow Us