The Multifaceted Role of STAT3
| Signal transducer and activator of transcription 3 (STAT3) protein belongs to a group of intracellular transcription factors that mediate a variety of functions such as cellular differentiation, proliferation, and apoptosis in various contexts. Under normal conditions, STAT3 activity is transient and tightly regulated. However, STAT3 is also considered an oncogene since its constitutive and aberrant regulation or hyperactivation has been associated with different types of tumors. This hyperactivity has been linked to many factors such as excessive stimulation by cytokines or growth factors, and loss of negative regulation of STAT3 (2). |
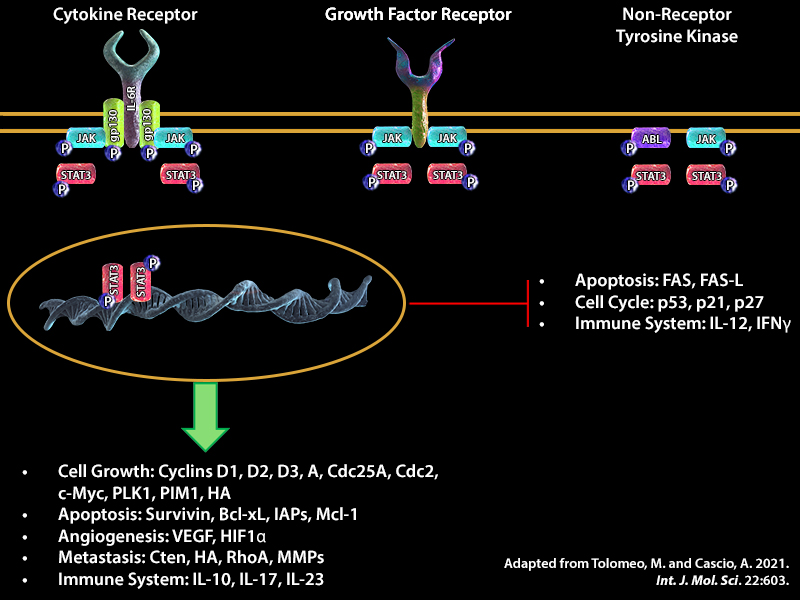 |
| STAT3 is activated by a number of extracellular signaling proteins such as growth factors VEGF and EGF. Binding of these factors to their respective receptors VEGFR and EGFR initiates signaling cascades that involves phosphorylation and activation of protein kinases in the cytoplasm, which in turn can phosphorylate STAT3. Phosphorylation of STAT3 on residue 705 (Y705) is crucial for its dimerization, nuclear translocation, and DNA binding activity. STAT3 can also be phosphorylated on serine residue 727 (S727). Phosphorylation at this second site may enhance transcriptional activity of STAT3 (1). Once in the nucleus, STAT3 can bind to interferon-stimulated response element (ISRE) or gamma interferon-activated sequence (GAS) promoter regions and together, with co-factors such as Nuclear Factor Kappa (NF-κB) and Hypoxia-inducible factor 1 alpha (HIF-1α), can induce transcription of genes that are involved in inflammation, cell proliferation, and survival (1). Due to the tumorigenic potential of STAT3, these factors could trigger aberrant cell growth and cell cycle transition, angiogenesis, invasion, and tumor metastasis, which leads to modulation of the expression of the proteins involved in these pathways. Some examples are alterations in the expression of the following proteins: B-cell lymphoma 2 (Bcl-2) an anti-apoptotic protein that regulates mitochondrial dynamics, cyclins D1-3 and cyclin A, proteins involved in G1 to S phase transition, and Matrix metalloproteinase 9 (MMP-9) a protein that breaks down the extracellular matrix, contributing to invasion and metastasis. |
 Structure of STAT3 Wikimedia Commons. |
In addition to its role in cancer, STAT3 can also have pro- or anti-viral functions. These opposing functions likely depend on a range of factors including differing roles of STAT3 in different host cell types, and virus-specific requirements such as promoting cell survival to enable replication or inducing cell lysis to release virus progeny. For instance, depending on the cell type, hepatitis B virus (HBV) promotes STAT3 activation to upregulate pro- or anti-apoptotic genes (3). |
| Aberrant STAT3 function has also been implicated in COVID-19-related lung damage. In a recent publication, a mechanism for SARS-CoV-2 pathophysiology was proposed. The authors suggested a link associated with a dysfunction in STAT1 and compensatory hyperactivation of STAT3, which leads to upregulation of plasminogen activator inhibitor-1 (PAI-1) (4). In infected cells, upregulated PAI-1 binds to toll-like receptor 4 (TLR4) on macrophages, induces the secretion of proinflammatory cytokines and chemokines, and leads to the recruitment and activation of innate immune cells. This local inflammation in the affected lung area causes the destruction of the lung architecture, and subsequent infection of the regional endothelial cells and the induction of a hypoxic environment driving PAI-1 production. In addition, EGFR is activated in the injured lung resulting in phosphorylation of STAT3. Therefore, the positive feedback loop between STAT3 and PAI-1 ultimately leads to widespread lung damage. |
| BioLegend Reagents for STAT3 and STAT-related Research BioLegend offers a number of reagents and primary antibodies specific for signaling proteins, transcription factors and regulators, including STATs (such as STAT3) in both phosphorylated and unphosphorylated states. Many of our antibodies are available in multiple formats and have been validated in various applications including intracellular flow cytometry. This allows multi-parameter analysis of individual populations within a complex population of cells such as peripheral blood mononuclear cells. |
| Specificity | Clone(s) | Reactivity | Application |
|---|---|---|---|
| EGFR Phospho (Tyr845) | QA19A93 | Human | WB |
| STAT1 | 10C4B40 | Human | IP, WB |
| STAT1 Phospho (Ser727) | A15158B | Human, Mouse | ICFC, ICC, WB |
| STAT1 Phospho (Tyr701) | A17012A | Human | ICFC, ICC, ChIP, WB |
| STAT2 | Poly6245 | Human | IP, WB |
| STAT3 | 15H2B45 | Human | ICFC |
| STAT3 | 4G4B45 | Human, Mouse | ICFC, ICC, WB |
| STAT3 Phospho (Ser727) | A16089A | Human, Mouse | ICC, WB |
| STAT3 Phospho (Ser727) | A16089B | Human | ICFC, ICC, ChIP, WB |
| STAT3 Phospho (Tyr705) | 13A3-1 | Human, Mouse | ICFC, WB |
| STAT4 Phospho (Tyr693) | A19016A | Human | WB |
| STAT5 | 9C8B50 | Human, Mouse | ICC, IP, WB |
| STAT5 Phospho (Tyr694) | A17016B.Rec | Human | ICFC, AP, WB |
| STAT6 | 16G12A08 | Human, Mouse | ICC, IP, ChIP, WB |
| STAT6 Phospho (Tyr641) | A15137A | Human | ICC, ChIP, WB |
| STAT6 Phospho (Tyr541) | A15137E | Human | ICFC, WB |
 Login / Register
Login / Register 






Follow Us