Th17 Polarization of Mouse CD4+ Cells
Introduction:
T helper 17 (Th17) cells are a subset of CD4+ T helper cells characterized by their production of IL-17, particularly IL-17A and IL-17F. They are involved in autoimmune diseases such as multiple sclerosis, psoriasis, autoimmune uveitis, rheumatoid arthritis, and juvenile diabetes, but are also important in anti-microbial defenses at epithelial/mucosal barriers, particularly against Candida and Staphylococcus. In addition to IL-17, Th17 cells also produce IL-21 and IL-22 that contribute to pro-inflammatory responses. The transcription factors RORα and RORγt have been identified as important factors in the differentiation of these cells. Th17 cells can be induced in vitro by a variety of cytokines including TGF-β, IL-6, IL-21 and IL-23. Here we provide an effective protocol for the generation of mouse IL-17 producing cells in vitro.
Isolation of CD4+ Cells From Lymph Nodes:
- Harvest lymph nodes (superficial cervical, mandibular, axillary, inguinal, and mesenteric) from mice.
- Tease lymph nodes through a sterile 70-µm nylon cell strainer to obtain single-cell suspensions in complete RPMI containing 10% FCS and 0.05 mM 2-ME (complete medium).
- Resuspend cells in complete medium and use your favorite method to isolate CD4+ cells, such as the MojoSort™ Mouse CD4 T Cell Isolation Kit.
Th17 Polarization of CD4+ Cells:
- On day 0, coat 60 × 15 mm of plastic petri dishes with anti-mouse CD3ε, clone 145-2C11 (5 µg/ml). Incubate at 37°C for 2 hours or 4°C overnight. Aseptically decant antibody solution from the plate. Wash plate 3 times with sterile PBS. Discard liquid.
- Plate CD4+ cells at 10 x 106/5 ml/dish. Culture cells for 4 days in the presence of anti-mouse CD28, clone 37.51 (5 µg/mL), recombinant mouse IL-6 (50 ng/mL), recombinant human TGF-β1 (1 ng/mL), recombinant mouse IL-23 (5 ng/ml), anti-mouse IL-4 (10 µg/mL), and anti-mouse IFN-γ (10 µg/mL).
- On day 3, slowly add 5 ml of fresh media along with same the concentration of antibodies/cytokines as used on day 0.
- On day 4, wash cells once and then restimulate in complete medium with 500 ng/ml PMA and 500 ng/mL ionomycin, in the presence of Brefeldin A (If you are looking for IL-21 production, use monensin) for 4 - 5 hours.
- After harvesting, the cells are ready for staining.
*Note: recombinant human TGF-β is effective for stimulating mouse cells.
Reagent List:
- Sterile PBS
- Cell culture medium (RPMI 1640 supplemented with 10% FBS and 0.05 mM 2-ME)
- Sterile plastic petri dishes
- RBC Lysis Buffer (Cat. No. 420301)
- Anti-mouse CD3ε Antibody, clone 145-2C11 (Ultra-LEAF™ format, Cat. No. 100340)
- Anti-mouse CD28, clone 37.51 (Ultra-LEAF™ format, Cat. No. 102116)
- Anti-mouse IL-4, clone 11B11 (Ultra-LEAF™ format, Cat. No. 504122)
- Anti-mouse IFN-γ, clone XMG1.2 (Ultra-LEAF™ format, Cat. No. 505834)
- Recombinant mouse IL-6 (carrier-free) (Cat. No. 575704)
- Recombinant mouse IL-23 (carrier-free) (Cat. No. 589002)
- Recombinant human TGF-β1 (carrier-free) (Cat. No. 781802)
- Brefeldin A (Cat. No. 420601)
- Monensin Solution (Cat. No. 420701)
- PMA (Phorbol 12-myristate 13-acetate) (Cat. No. P8139 from Sigma)
- Ionomycin (Cat. No. I0634 from Sigma)
References:
- Cua DJ. et al. 2010. Nat Rev Immunol. 10: 479.
- Pappu R. et al. 2010. J Clin Immunol. 30:185.
- Kennedy, J. et al., 1996. J. Interferon Cytokine Res. 16: 611.
- Schubert, D. et al., 2004. J. Immunol. 172: 4503.
- Infante-Duarte, C. et al., 2000. J. Immunol. 165: 6107.
- Harrington, L. E. et al., 2005. Nature Immunol. 6:1123.
- Nekrasova, T. et al., 2005. J. Immunol. 175: 2734.
- Yen, D. et al., 2006. J. Clin. Invest. 116: 1310.
- Ehirchiou, D. et al., 2007. J. Exp. Med. 204:1519.
- Kang, S. G. et al., 2007. J. Immunol. 179:3724.
- Harrington, L. E. et al., 2005. Nature Immunol. 6:1123.
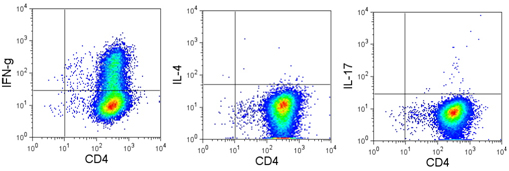
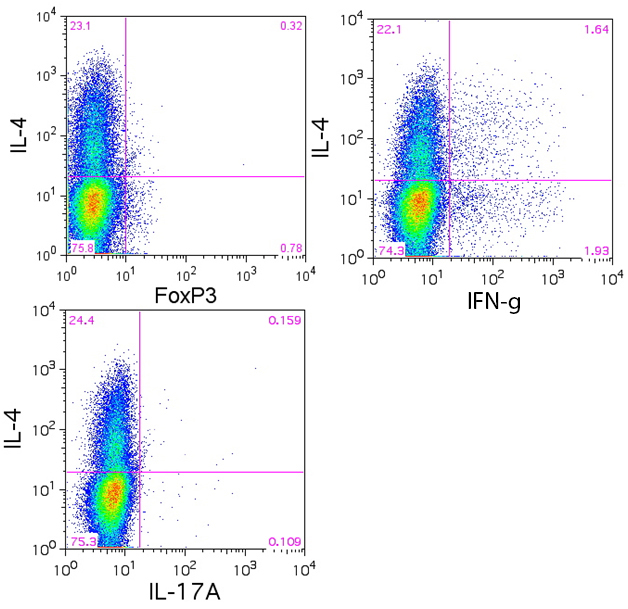
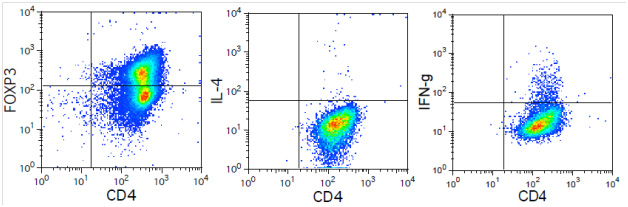
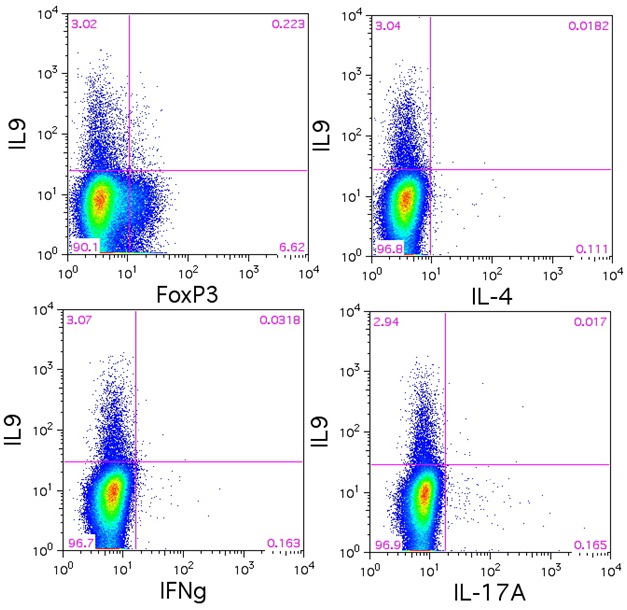
Data:

Naïve BALB/c mice CD4+ T cells were polarized with 5 µg/ml plate-bound anti-mouse CD3ε (clone 145-2C11, Cat. No. 100339), 5 µg/ml soluble anti-mouse CD28 (clone 37.51, Cat. No. 102115), 50 ng/ml mouse IL-6 (Cat. No. 575704, 5 ng/ml mouse IL-23 (Cat. No. 589002), 1 ng/ml TGF-β1 (Cat. No. 781802), 10 µg/ml anti-mouse IL-4 (clone 11B11. Cat. No. 504121), 10 µg/ml anti-mouse IFN-γ (clone XMG1.2, Cat. No. 505833) for 4 days. On day 3, 5 ml of fresh media was added along with same the concentration of antibodies/cytokines as used on day 0. On day 4, the cells were re-stimulated with 500 ng/ml PMA/ionomycin in the presence of BFA (if detecting IL-21, monensin is recommended instead) for 6 hours. Then the cells were harvested and surface stained with CD4 FITC (clone GK1.5, Cat. No. 100405), intracellular stained with IL-17 PE (clone TC11-18H10.1, Cat. No. 506904) along with IFN-γ APC (clone XMG1.2 , Cat. No. 505809), IL-4 APC (clone 11B11, Cat. No. 504106), IL-9 APC (clone RM9A4, Cat. No. 514105) , IL-21 Alexa Fluor® 647, or IL-22 Alexa Fluor® 647 (clone Poly5164, Cat. No. 516406) after fixation and permeabilization.
 Login / Register
Login / Register 







Follow Us