Ubiquitin as an IHC Marker for Neurodegeneration
Ubiquitin is a small regulatory protein that is expressed in all eukaryotic cells. Ubiquitination, a process whereby ubiquitin is attached to a substrate protein, is involved in many cellular processes including protein degradation, endocytic trafficking, antigen processing, organelle biogenesis, transcriptional regulation, and DNA repair. Aberrant ubiquitination and/or dysfunction in ubiquitin-mediated pathways have been linked to numerous disorders.
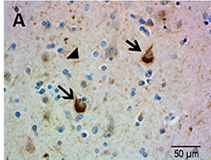
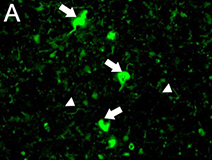
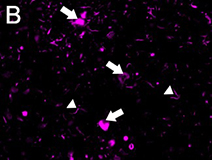
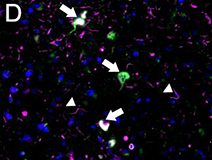
Ubiquitin immunostaining is often among the basic immunohistochemical (IHC) panels for neurodegeneration detection. Ubiquitin accumulates in morphologically distinct deposits associated with several neurodegenerative disorders, such as Tauopathies, Alzheimer’s (AD) and Parkinson’s (PD) diseases. These disorders are collectively called proteinopathies and share a common feature: abnormal misfolding of key proteins that increases their tendency to self-aggregate and form proteinaceous inclusions. Loss of normal function, resistance to degradation and clearance, and interference with normal cellular processes are attributed to the aggregated state of proteins involved.
Antibodies against ubiquitin can visualize the presence of disease-related structures, such as cytoplasmic or extracellular inclusions. The use of disease specific antigens or biomarkers can supplement data obtained from ubiquitin staining to further distinguish among pathologies. Some examples are highlighted in the table below.
 Login/Register
Login/Register 








Follow Us