Immunofluorescence vs. Immunocytochemisty vs. Immunohistochemistry...What IS the difference?
 |
|
The term immunofluorescence is often confused with immunocytochemistry and immunohistochemistry. All three of these terms are frequently used interchangeably, and this can lead to confusion when looking to purchase an antibody to use in your microscopy experiment. |
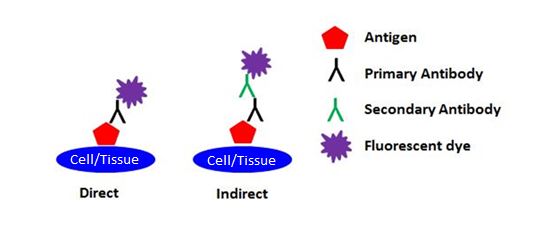
| Immunofluorescence (IF) refers to the detection method being used (i.e., the use of fluorescent dyes to visualize markers of interest), and has nothing to do with the sample type being used. IF can further be broken down into two categories: direct and indirect. Direct IF uses a single antibody that is directly conjugated to a fluorescent dye in order to detect the target of interest. Indirect IF uses two antibodies to detect the target of interest. The primary antibody that binds the target is unconjugated, and the secondary antibody that reacts with the primary antibody is conjugated to a fluorescent dye. |
 |
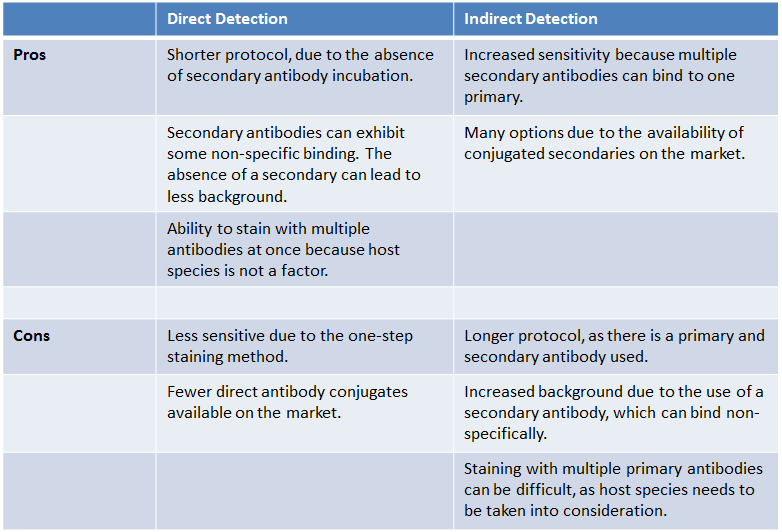
| What are the pros and cons of each detection method? |
 |
Immunocytochemistry (ICC) |
|
Immunocytochemistry is most commonly performed on cells that have been grown in a monolayer in a culture dish or transferred from suspension to a slide for viewing on a microscope. When working with adherent cells, the cells are often grown directly on a coverslip. Depending on the cell type, the cells may not adhere to the coverslip on their own and the coverslips will need to be coated with gelatin or either poly-L-lysine or poly-D-lysine in order to aid in this process. Suspension cells are usually fixed by adding the fixative directly to the cell culture media for approximately 15-20 minutes, washed, and resuspended in buffer and directly placed on a gelatin or poly-L/poly-D-lysine coated slide, and smeared using the edge of another slide or the side of a pipet tip. Suspension cells can also be immobilized to slides using a centrifugation method known as Cytospin™.
|
 |
|
The most common permeabilizing reagent used for immunocytochemistry is Triton X-100, ranging from 0.1-0.5% in PBS, but saponin, digitonin, and Tween-20 can also be used. When using organic solvents as the fixative, a permeabilization step is usually not necessary. |
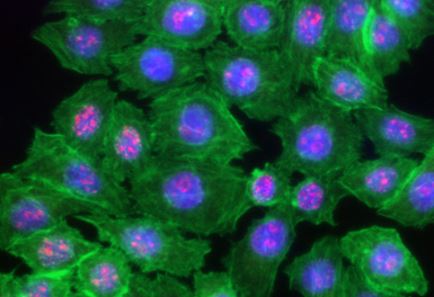 |
HeLa cells were fixed with 1% paraformaldehyde (PFA) for 10 minutes, permeabilized with 0.5% Triton X-100 for 10 minutes and blocked with 5% FBS for 30 minutes. Then the cells were intracellular stained with 2.5 μg/ml of Ki-67 (clone Ki-67) Alexa Fluor® 594 (red) in blocking buffer overnight at 4°C and followed by Alexa Fluor® 488 Phalloidin (green) staining for 20 minutes. Nuclei were counterstained with DAPI and are shown in blue. The image was captured with 40x objective |
| Organic solvents allow for the preservation of cellular architecture, but they also dehydrate the samples, precipitate proteins, denature the protein you are hoping to detect, denature GFP and other fluorescent proteins, and remove lipid-linked proteins and other soluble molecules. Organic solvents work well when staining for cytoskeletal markers. | 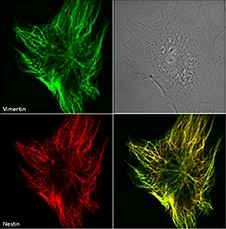 Immunofluorescence of Clone Nestin 20. Nestin is stained in red and Vimentin in green on methanol fixed mouse embryonic cells. Photo courtesty of R. Goldman, PhD, Northwestern University |
You can find our general immunocytochemistry protocols using paraformaldehyde (cross-linking) or methanol (organic solvent) on our website in our Technical Protocol section. Be sure to also check out our brand new Immunocytochemistry Protocol Video. |
| Immunohistochemistry (IHC) |
| Immunohistochemistry is most commonly performed on tissue samples that have either been frozen in a cryopreservation media such as optimal cutting temperature (OCT) compound or fixed in neutral buffered formalin and embedded in paraffin. Immunohistochemistry allows you to visualize your target of interest in tissue samples, while maintaining cellular structure. |
| Immunohistochemistry- Frozen Tissues (IHC-F) |
| Preparing frozen tissues for microscopy most often requires snap freezing it in either liquid nitrogen or iso-pentane. Before sectioning the tissue, it is commonly placed in a mold and covered in OCT embedding medium. Once the tissue is embedded, you can move it to the cryostat and begin sectioning. Generally, 5-20 μm sections are made and these are mounted to slides that have been coated with gelatin or poly-L/poly-D-lysine, as with immunocytochemistry. You can also place the freshly harvested tissue directly into a mold and cover with OCT and then place the mold containing the embedded tissue directly into liquid nitrogen. The tissue block can be stored at -80°C until you are ready for sectioning. Once ready for sectioning, the frozen tissue block can be transferred to the cryostat, sections made, and mounted onto coated slides as above. Slides can be stored at -80°C up to a year until ready for staining. When ready for use, slides are removed from the freezer and usually fixed with methanol or acetone; therefore no antigen retrieval step is necessary as with formalin fixed paraffin embedded (FFPE) tissues. |
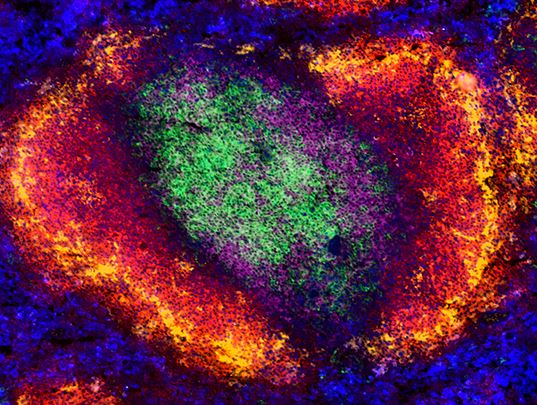 |
C57BL/6 mouse spleen tissue stained for CD4, CD8a, B220, CD169, and F4/80. View additional details and single stain data. |
Immunohistochemistry- Paraffin Embedded (IHC-P) |
| Paraffin embedding of tissues is ideal for preserving tissue morphology. If maintaining the tissue morphology is absolutely critical due to the markers being stained for, it can be a good idea to perform cardiac perfusion on your animals with phosphate buffered saline, immediately followed by 10% formalin or 4% paraformaldehyde as soon as most of the blood has been flushed from the animal. Once perfusion is complete, the desired tissues and organs are harvested and placed in a fixative and stored on ice until ready to embed. While perfusion of the animal is recommended since blood is highly autofluorescent, for standard stains where morphology is not as critical, harvested tissues can be placed into a 10% formalin or 4% paraformaldehyde solution until ready to embed. Prior to starting the paraffin embedding process, the tissue is left in fixative anywhere from 4-48 hours. However, be aware of over-fixing the tissue, as this can cause issues with epitope availability (masking) and decrease antibody binding. |
| The act of embedding the tissue in paraffin is an involved process and requires one to first dehydrate the tissue with a series of alcohol immersions that increase in concentration. Paraffin is not water soluble, and this ensures that the water that is in the tissue is slowly replaced by the alcohol. As paraffin is not soluble in alcohol either, the next step in the process is called clearing, and this refers to the solvents that are used in this process. The alcohol that has replaced the water and dehydrated the tissue is replaced by an organic solvent, most commonly xylene, which makes the tissue receptive to the paraffin. As the name implies, clearing also makes the tissue clear or transparent. The tissue is now ready to be embedded in warm paraffin, which fills the space previously occupied by the water. Once the paraffin cools, the tissue hardens and is ready to be sectioned using a microtome. Sections of approximately 5-8 μm are cut and mounted on glass slides. As most antibody staining solutions are aqueous, you now need to remove the paraffin and replace the water that you just removed! In order to do this, you basically repeat the dehydration and clearing steps in reverse. The slides are deparaffinized by passing through xylene and then decreasing amounts of alcohol. |
 |
| Antigen Retrieval Methods |
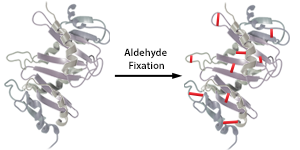
| Due to the crosslinking fixatives used in the paraffin embedding protocol, antibodies may not be able to bind due to epitope masking. In order to make the binding epitope available, or unmask it, a process called antigen retrieval needs to be performed. Antigen retrieval reverses the crosslinked proteins, thereby allowing the antibody access to the epitope it needs to bind to. There are generally two types of antigen retrieval methods: Heat Induced Epitope Retrieval (HIER) and Proteolytic Induced Epitope Retrieval (PIER). |
| Heat Induced Epitope Retrieval (HIER) |
| HIER is the most common type of epitope retrieval that is used as the protocol is more convenient than PIER. There are several important factors that need to be tested and optimized in order to find the appropriate conditions for the antigen you are working with. The incubation time, pH and components of buffer, and temperature are all things to take into account when developing your protocol. The most common buffers used in HIER are either a low pH (~6.0) sodium citrate or a high pH (~9.0) Tris/EDTA used at a temperature of ~95-100°C for 10 min. Buffer conditions, temperatures, and incubation times should be carefully optimized, and may need to be determined for each experiment depending on the antigen. |
 |
Human paraffin-embedded colon tissue slices were prepared with a standard protocol of deparaffinization and rehydration. Antigen retrieval was done with Citrate Buffer, pH 6.0 at 95°C for 40 minutes. Then, the tissue was stained with 10 μg/mL of Alexa Fluor® 647 anti-Vimentin (clone O91D3) antibody (green) and 10μg/mL of Alexa Fluor® 594 anti-CD66d/e (clone 308/3-3) antibody (red) over night at 4°C. Nuclei were counterstained with DAPI (blue). |
Proteolytic Induced Epitope Retrieval (PIER) |
| Some antigens may require a little harsher method in order to unmask the epitope and make it available for antibody binding. PIER uses enzymes in order to digest the protein cross-links that were formed during fixation. The most common proteolytic enzymes used are trypsin, pronase, and Proteinase K. As with HIER, conditions need to be tested in order to find the optimal conditions for antigen retrieval. The incubation time, temperature, and concentration of proteolytic enzyme used are all critical. Hopefully after reading this blog, you have a better understanding of the differences between IF, ICC, and IHC. The take home message is that immunofluorescence is a detection method, and has nothing to do with the type of samples you may be working with. |
| BioLegend is aware of the confusion with the terminology, and we are currently working on updating all of our technical data sheets (TDS) to reflect whether a product can be used in ICC, IHC-F, or IHC-P. The term immunofluorescence (IF) will eventually be removed from all TDS’s. We always encourage checking the TDS to see which applications we’ve quality tested or validated and what has been reported in the literature. You can find more reagents, tips, and protocols with our Microscopy Webpage. As always, feel free to contact our tech support group at tech@biolegend.com if you have any questions!! |
|
Contributed by Kellie Johnson.. |
 Login/Register
Login/Register 






Follow Us