Advancing Alzheimer’s Research With Our Immunoassays
Alzheimer’s disease (AD) and related forms of dementia are increasingly affecting the aging population worldwide. The World Alzheimer’s Report indicates around 50 million people are affected by AD1, which is only projected to increase if interventions and treatments are not successful. While new biomarkers are currently being developed for aggregation, inflammation, and synaptic dysfunction, treatments for the disease are limited2.
For Alzheimer’s Awareness Month, we’re highlighting the resources our scientists have developed to advance AD research, including high-quality and specific immunoassays for the detection of key target proteins involved in the pathogenesis of Alzheimer’s disease.
To learn more about the known associated proteins that have been implicated in AD, and a comprehensive list of available reagents, explore our Neurodegeneration resources.
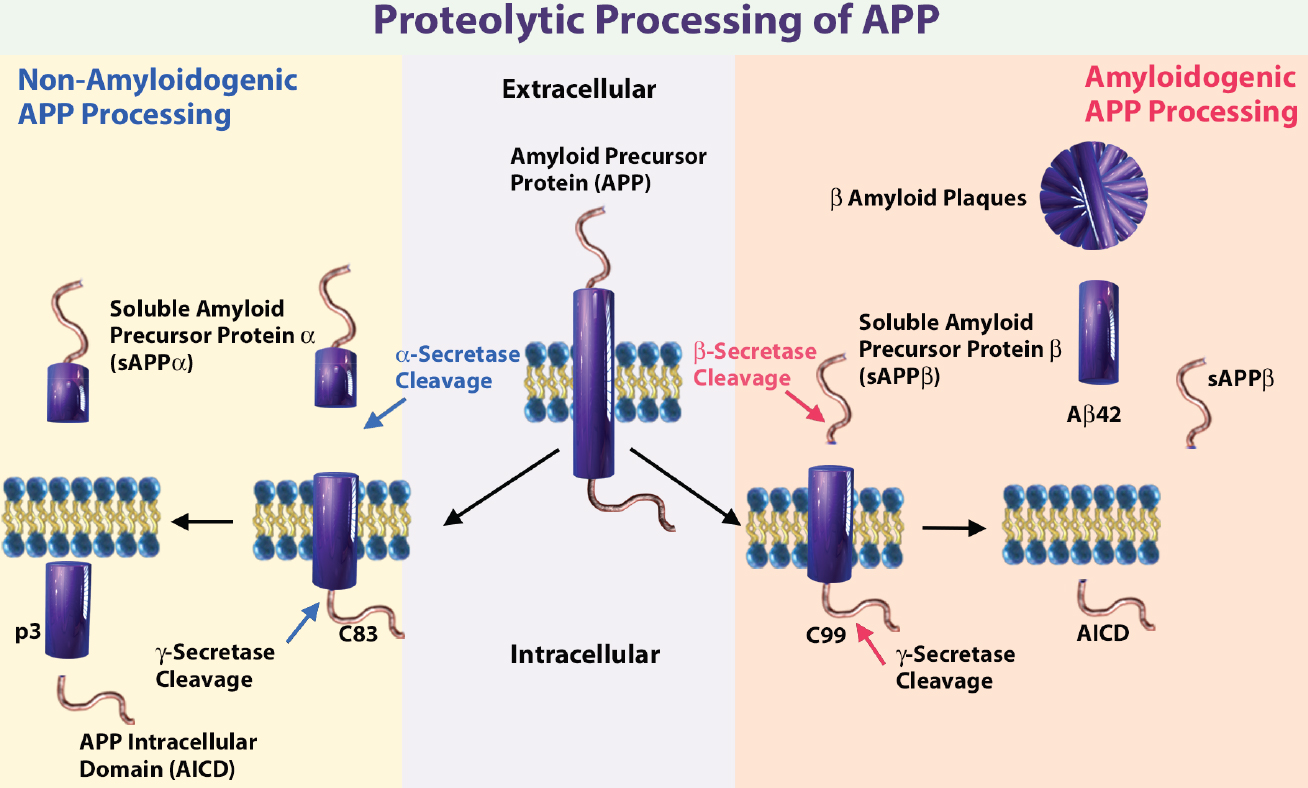
Schematic illustrating the sequential cleavage steps in APP processing. Get a more detailed description on APP processing. Abbreviations: 83-amino-acid C-terminal APP fragment (C83), APP intracellular domain (AICD), and membrane-tethered C-terminal fragments β (CTFβ or C99).
Amyloid beta peptide (Aβ) is produced through the proteolytic processing of a transmembrane protein, amyloid precursor protein (APP), by β- and γ-secretases. Aβ accumulation in the brain is proposed to be an early toxic event in Alzheimer's disease pathogenesis. There are several known amyloid isoforms generated by APP cleavage, including amyloid beta (1-42), amyloid beta (1-40)3.
The accumulation of amyloid β peptide (1-42) in extracellular plaques is one of the hallmarks of AD. Although Aβ (1-40) is more abundant than Aβ (1-42), it has a lower tendency to aggregate. Aβ42/Aβ40 ratio in cerebrospinal fluid (CSF) is an important diagnostic biomarker of the severity of disease6,9. A lower Aβ42/Aβ40 (Aβ42/40) plasma ratio is associated with higher amyloid cortical burden, greater cognitive decline and increased risk of developing AD dementia10,11.
Our LEGEND MAX™ Human Amyloid β 1-42 and Amyloid β 1-40 ELISA Kits are an important tool to determine the Aβ42/Aβ40 and are designed for the accurate quantitation of human amyloid protein fragments. Both LEGEND MAX™ ELISAs are analytically validated and can be used to detect native proteins in several types of human samples, including serum, plasma, and CSF.
Our sampler kits are ideal for visualization of native and post-translationally modified forms of proteins implicated in neurodegeneration, and provide accurate detection of these species. The Aβ Antibody Sampler Kit offers flexibility for sampling and detection of full-length APP and various APP cleavage fragments including Aβ (1-40) and Aβ (1-42) peptides.

Aβ42/Aβ40 ratio is associated with disease severity.
We also provide reagents for other proteins implicated in AD, such as tau. In normal physiology, tau is an important protein in microtubule stability and structure. Hyperphosphorylation of tau is one of the leading causes of reduction in binding affinity and its dissociation from microtubules. This affects the structural integrity of axons, and excess levels of tau lead to abnormal aggregation and the formation of insoluble fibrils and tangles.

Our Tau Antibody Sampler Kit is designed to measure modified and unmodified tau species including tau phosphorylated at serine 262, which is commonly associated with AD legion sites. It also detects tau phosphorylated at threonine 181, which has been shown to have diagnostic utility for several neurological disorders including AD5,7.
Our LEGENDplex™ Human Neurodegenerative Disease Biomarker Panel is designed to measure five neurodegenerative biomarkers simultaneously (α-Synuclein, Tau, Aβ40, Aβ42, and Neurofilament-L). This panel is a multiplex bead-based immunoassay that utilizes fluorescence-encoded beads suitable for use on various flow cytometers. It provides higher detection sensitivity with broader dynamic ranges than the traditional ELISA method.
In addition to these immunoassays, we are continuously expanding our portfolio to include aggregate-preferring antibodies and phospho-specific antibodies for tau and amyloid beta, as well as antibodies for characterizing cell signaling of various cell types in the brain.
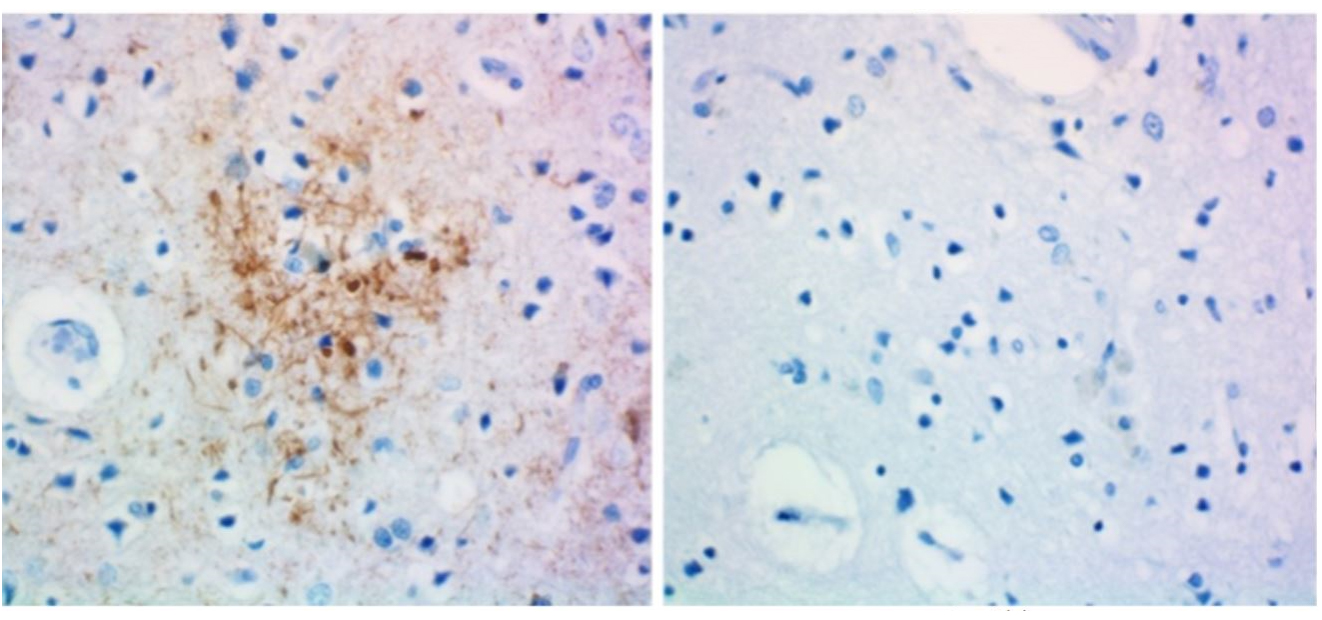
IHC staining of anti-tau, 1-223 antibody (clone A16103A) and rat IgG2b isotype control on formalin-fixed paraffin-embedded Alzheimer's disease brain tissue. Biotinylated anti-rat IgG, HRP Streptavidin, and DAB (3,3'-diaminobenzidine) substrate were used as the detection system. Slides were counterstained with hematoxylin.
BioLegend is committed to empowering Alzheimer’s and neurodegenerative disease research by providing resources for key targets, such as tau, APP and Aβ. Discover the difference with our high-quality and validated reagents created by our expert scientists.
References
- Alzheimer's Association. 2019. Alzheimer's disease facts and figures. Alzheimer's & dementia, 15(3), 321-387. DOI:10.1016/j.jalz.2019.01.010
- Long, J. M., & Holtzman, D. M. 2019. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell, 179(2), 312–339. DOI:10.1016/j.cell.2019.09.001
- Zhang, Y et al. 2011. APP processing in Alzheimer's disease. Molecular brain vol. 4 3. DOI:10.1186/1756-6606-4-3
- Jin, S et al. 2016. Amyloid-β(1-42) Aggregation Initiates Its Cellular Uptake and Cytotoxicity. The Journal of biological chemistry vol. 291,37: 19590-606. DOI:10.1074/jbc.M115.691840
- Guzman-Martinez, L et al. 2019. Biomarkers for Alzheimer's Disease. Current Alzheimer research vol. 16,6 (2019): 518-528. DOI:10.2174/1567205016666190517121140
- Hansson, O., Lehmann, S., Otto, M. et al. 2019. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alz Res Therapy 11, 34. DOI:10.1186/s13195-019-0485-0
- Guo, J. L., Narasimhan, S., Trojanowski, J. Q., & Lee, V. M. et al. 2016. Unique pathological tau conformers from Alzheimer's brains transmit tau pathology in nontransgenic mice. The Journal of experimental medicine, 213(12), 2635–2654. DOI:10.1084/jem.20160833
- Kim, K., et al. 2020. Clinically accurate diagnosis of Alzheimer's disease via multiplexed sensing of core biomarkers in human plasma. Nature communications, 11(1), 119. DOI:10.1038/s41467-019-13901-z
- Korecka, M. et al. 2020. Alzheimer’s Disease Neuroimaging Initiative. Analytical and Clinical Performance of Amyloid-Beta Peptides Measurements in CSF of ADNIGO/2 Participants by an LC-MS/MS Reference Method. Clinical chemistry, 66(4), 587–597. DOI:10.1093/clinchem/hvaa012
- Baldeiras, I. et al. 2018. Addition of the Aβ42/40 ratio to the cerebrospinal fluid biomarker profile increases the predictive value for underlying Alzheimer's disease dementia in mild cognitive impairment. Alzheimer's research & therapy, 10(1), 33. DOI:10.1186/s13195-018-0362-2
- Lehmann, S. et al. 2020. Cerebrospinal fluid A beta 1-40 peptides increase in Alzheimer's disease and are highly correlated with phospho-tau in control individuals. Alzheimer's research & therapy, 12(1), 123. DOI:10.1186/s13195-020-00696-1
- Crescenzi, O. et al. 2002. Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur J Biochem 269: 5642-5648. DOI: 10.1046/j.1432-1033.2002.03271.x PDB DOI:10.2210/pdb1IYT/pdb
- Cerofolini, L., et al. 2020. Mixing A beta (1-40) and A beta (1-42) peptides generates unique amyloid fibrils. Chem Commun (Cambridge, England). 56: 8830-8833. DOI: 10.1039/d0cc02463e PDB DOI:10.2210/pdb6TI6/pdb
 Login/Register
Login/Register 






Follow Us