Advancements in using iNKTs for Cancer Immunotherapy
| Immunotherapy harnesses the body's own immune system to provide anti-tumor responses and fight cancer. There are several immunotherapy-based treatments that have been approved for clinical use and many others that are still being developed. Previously, on the blog, we've looked at immune checkpoint inhibitors and CAR-T cells. In this blog, we'll focus on research being done to develop immunotherapies that exploit a specific immune cell - Invariant NKT cells (iNKTs). |
What are iNKT cells? |
|
Like their name suggests, NKT cells have characteristics of NK and T cells and express both a T cell receptor complex and many canonical NK cell markers. While there are a few different subsets of NKT cells, for the purpose of this blog, we'll focus on iNKTs. iNKT cells express an invariant TCR with a limited repertoire that responds to the presentation of glycolipids by a class-I related cell surface molecule, CD1d. Upon stimulation, they are able to release a broad range of cytokines to elicit adaptive and innate immune responses, activate other immune cell populations, and induce dendritic cell maturation. Learn more about NKT cell development and function on our NK and NKT cell webpage!
|
| iNKT cells can recognize tumor cells both indirectly and directly, either by recognizing CD1d on antigen-presenting cells in the tumor microenvironment or CD1d expressed by the tumor cell. In addition to cytokine release, iNKT cells secrete cytotoxic molecules including perforin, granzymes, and Fas ligand which can cause cytolysis of tumor cells that express CD1d. Because iNKT cells bridge both arms of the immune system, are cytotoxic, and are able to produce inflammatory cytokines, they are very attractive targets for developing immunotherapies. In fact, there have been several studies correlating increased frequency/activity of iNKT cells with overall survival in a number of different tumor types (reviewed in 6, 9). A role for iNKTs in tumor clearance has been well-documented in mouse models, and adoptive transfer of iNKTs using mouse tumor models also suggests that iNKTs can contribute to an anti-tumor response (1). | 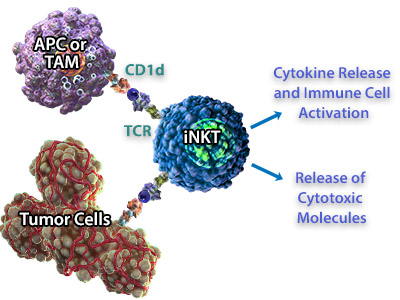 iNKT cells can recognize CD1d expressed on Antigen Presenting Cells (APC) or Tumor-Associated Macrophages (TAMs). In response, they can release cytokines to activate other immune cells like NK cells, and release cytotoxic molecules for direct killing of the tumor cells. |
Stimulation with α-GalCer |
| Functionally, iNKT cells respond to the α-GalCer glycolipid bound to CD1d. Initial clinical trials concentrated on administration of α-GalCer to stimulate iNKT cells and increase cytokine production (3, 5, 7). Though administration of α-GalCer does not seem to have any major toxicity-related effects, one caveat to this method is that it relies on the quality and the quantity of the existing pool of iNKTs. Upon repeated stimulation with α-GalCer, there are fewer iNKT cells in circulation and cytokine production diminishes due to anergy of iNKTs. A number of strategies may help to circumvent these issues including using analogs of α-GalCer, directing their delivery by incorporating glycolipids into nanovectors, and optimizing the routes and schedule of administration of α-GalCer. Mouse models have suggested that adoptive transfer of α-GalCer loaded-APCs elicits a stronger immune response (reviewed in 6, 9). As such, in lieu of providing exogenous α-GalCer, some Phase I clinical trials have focused on adoptive transfer of monocyte-derived DCs which have been incubated with α-GalCer in culture. These treatments have resulted in increased numbers of activated iNKTs and downstream activation of B cells, T cells and NK cells. It is worth noting that while these studies have shown expansion of the iNKT cell populations, they typically show robust immunological responses in patients that have "normal"Â iNKT cell numbers prior to treatment. |
Adoptive Transfer of iNKT cells |
| As the treatments described thus far are dependent on the number of existing iNKT cells, another complementary approach to exploiting iNKTs for immunotherapy involves directly transferring activated iNKT cells into patients. Initial studies found that there were no toxic effects due to the adoptive transfer of stimulated iNKTs but the expansion of the iNKT cell population was transient. For these studies, PBMCs are typically stimulated with α-GalCer in culture; however, newer studies have utilized an antibody directed against the TCR Vα24-Jα18 (clone 6B11) to isolate iNKTs prior to stimulation and transfer, resulting in higher purity and a larger number of iNKTs that can be transferred (2). While these studies are promising, additional studies are necessary to optimize these treatment strategies to demonstrate efficacy. |
CAR-iNKT Therapies? |
| Chimeric-antigen receptor (CAR) T cell based immunotherapies have provided promising therapeutic options and many ongoing studies are working to develop CAR-iNKT-based therapies. CAR-iNKTs may be particularly exciting because iNKT cells have intrinsic anti-tumor responses through recognition of CD1d. Addition of a second recombinant TCR may give the cells multiple mechanisms for tumor recognition. Current studies have demonstrated that CARs can be successfully transduced into iNKTs using either a TCR that recognizes GD2 in neuroblastoma models or a CD19-specific CAR in mouse B cell lymphoma models (4, 8). Two of the major hurdles associated with CAR-T cell studies are tumor homing and graft vs. host disease (GvHD). Interestingly, in mouse models, CAR-iNKTs do not cause GvHD to the same extent as CAR-T cells and these studies have also suggested that tumor homing may be improved. Immunotherapies are constantly evolving and improving, and while many of the current therapies focus on manipulation of T cells, utilizing iNKTs may provide added benefits to anti-tumor responses. The ability of iNKT cells to both directly and indirectly contribute to tumor killing suggests that they may be exploited to treat a wide-range of tumors. While it is important to note the potential caveats associated with these strategies, there is promising research finding new ways to harness the power of iNKTs to fight cancer! |
|
Contributed by Kelsey Swartz, PhD. |
References:
|
 Login/Register
Login/Register 






Follow Us