3 Considerations for Intracellular Flow Cytometry (ICFC)
 |
| Designing an experiment for staining surface markers is pretty straight forward. For the most part, there are not specific buffer requirements when surface staining, and negative controls are usually restricted to unstained samples and isotype controls. If your area of research leads you to explore intracellular targets, there are additional things to consider before moving forward with your experiment. |
| 1. Choosing the appropriate concentration. |
| While using the optimal concentration in surface staining is important, doing so when performing intracellular staining is critical. The suggested amount of antibody for use in your flow experiment can be found on the Technical Data Sheets under 'Recommended Usage'. |
 |
| The recommended concentrations provided by BioLegend are determined by performing 3-5 point titrations and choosing the results that have the best signal to noise ratio. |
| While these concentrations provide a good starting point, there may be situations in which you will need to titer your antibodies to find the optimal concentration for your sample type. If you use too little antibody, you run the risk of not detecting a signal or not being able to distinguish between your negative and positive populations. If you use too much antibody, you could potentially start to see increased background staining that can lead to an entire cell population shifting, thereby leading to incorrect analysis of your data. This phenomenon is known as "intracellular shift". |
 |
| 2. Choosing the appropriate buffer system. |
| Staining for intracellular targets requires one to fix and then permeabilize the cells. The most common fixative used is paraformaldehyde (PFA) which stabilizes the cell membrane prior to permeabilization with a detergent or alcohol, allowing the antibodies directed against intracellular antigens to enter the cell and bind to their target. The location of your target of interest will determine the most appropriate buffer system for your experiment. |
| Cytoplasmic Targets |
Staining for cytoplasmic targets, such as cytokines, requires a two-step fixation and permeabilization protocol. For this, we offer two different options:
|
| While the fixation and permeabilization buffers (10X) are sold as separate products, our new Cyto-Fast™ Fix/Perm Buffer Set provides a fix/perm solution and perm wash solution (10X) that have been QC tested together as a set and they often improve the signal-to-noise ratio as compared to our traditional fixation and permeabilization buffers. This provides more consistent and reproducible results for your intracellular staining experiments. |
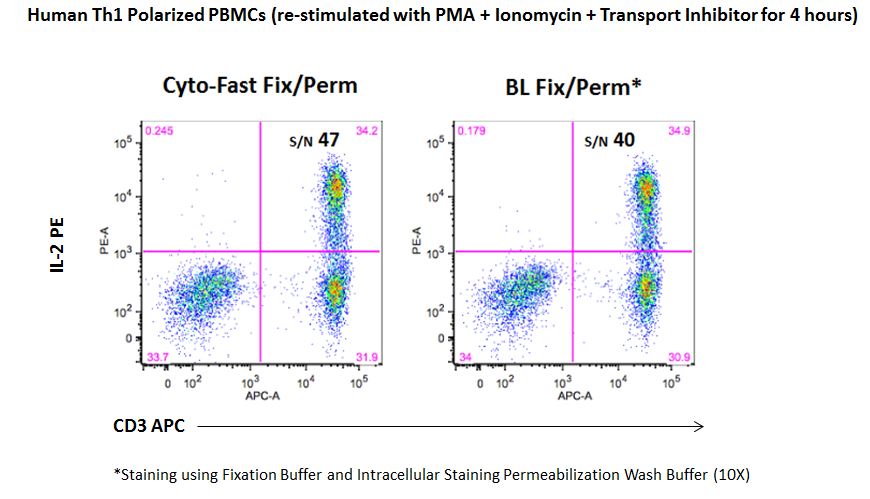 |
| Nuclear Targets |
| Staining for nuclear targets, such as transcription factors, requires a buffer system that will not only permeabilize the outer cell membrane, but also the nuclear membrane. In order to obtain optimal staining of transcription factors and other nuclear targets, we recommend using our True-Nuclear™ Transcription Buffer Set. This buffer is an improved version of our FOXP3 Fix/Perm Buffer Set. Some nuclear permeabilization buffers can have a detrimental effect on tandem dyes, thereby leading to a loss of FRET and increased compensation into the tandem dyes donor channel. |
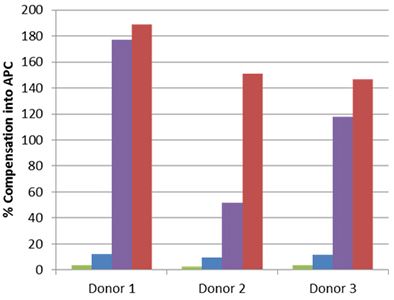 Whole blood was stained with human CD3 APC/Cyanine7 (blue and red) or APC/Fire™ 750 (green and purple), then lysed, washed and fixed under the following conditions: A) Fixed with FOXP3 Fix/Perm Buffer followed by permeabilization with FOXP3 Perm Buffer (purple and red). B) Fixed with True-Nuclear™ Fix Buffer, then permeabilized with True-Nuclear™ Perm Buffer (green and blue). Compensation requirements between APC and APC/Cyanine7 channels increased significantly for the FOXP3 Fix/Perm Buffer Set versus the True-Nuclear™ Transcription Factor Buffer set for both APC/Cyanine7 and APC/Fire™ 750. |
| Phosphorylated Targets |
| The use of phospho-specific antibodies for the analysis of phosphorylated proteins requires a buffer system that is a little harsher than the ones used for cytoplasmic and nuclear targets. We recommend staining phosphorylated proteins with our True-Phos™ Perm Buffer, which is methanol based, and specifically formulated for the detection of these phosphorylated proteins. |
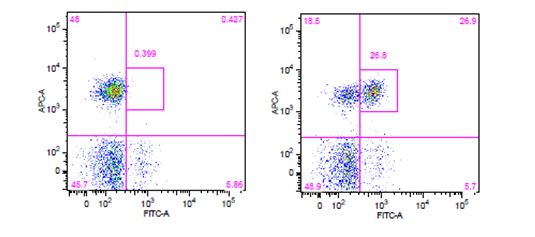 Human whole blood was stimulated with (right) or without (left) IL-6 for 15 minutes, then treated with RBC/Lysis Fixation Solution and permeabilized with True-Phos™ Perm Buffer, then stained with CD3 APC and STAT3 pY705 (clone 13A3-1) FITC. |
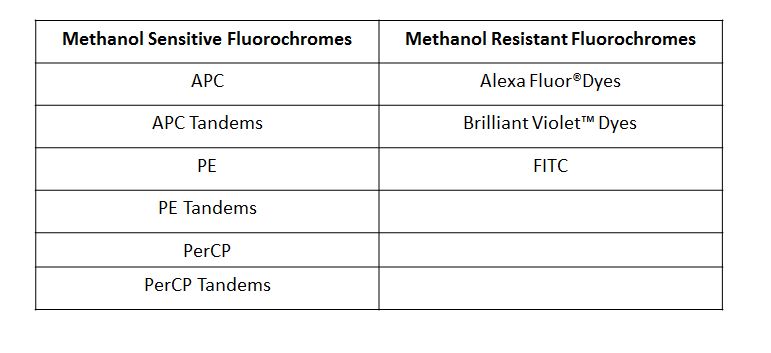
| Due to the fact that the True-Phos™ Perm Buffer is methanol based, special considerations need to be given to the choice of any antibodies used for surface staining. Methanol can alter or destroy the epitope that is recognized by an antibody, so it is good practice to do a small scale pilot study to determine if any of your surface stain antibodies can recognize a fixed epitope. You can also check our fixation page to see if we have tested a particular clone already. Please note, the clones on this page were evaluated using 4% PFA as the fixative, but it is a good source of information to give you an idea of whether your clone might stain a fixed epitope. If any of the clones you are working with do not recognize a fixed epitope, you can stain prior to using the True-Phos™ Perm Buffer if the conjugated fluorochrome is methanol resistant. |
 |
| "Special Consideration" Targets |
| There are some clones that march to the beat of their own drum so to speak, and in order to achieve optimal staining results, require their own intracellular staining protocol using buffers we haven’t covered yet. One example is our Ki-67 clones, Ki-67 and 16A8. For optimal staining, these clones require the use of ice cold 70% ethanol as a combined fixative-permeabilizing agent, and their specific protocol is outlined on the TDS. The moral of the story is to ensure you have chosen the appropriate buffer system for the intracellular targets you are looking to evaluate. |
| 3. Choosing the appropriate controls. |
| The use of the appropriate controls in your experiment is critical for being able to analyze your data correctly. You can learn more about controls with our Flow Controls webpage. |
| Isotype Controls |
| While isotype controls are a good choice when your experiments are limited to surface staining, it is generally not recommended they be used as the only control when performing intracellular staining. Occasionally when performing intracellular staining you can see shifting of the whole population, which can lead to incorrect analysis/assumption of what is actually staining. This is why isotype controls are generally used as a guide, and ideally should not be used as the ultimate criteria for setting the negative population, especially when there are clear cut positive and negative cell populations. |
| If you set the quadrants based on isotype control (black line), it appears as if you have more signal in Q1 and Q2. If you set the quadrants based on the inherent negative and positive populations (red line), you get a more accurate assessment of the signal in Q1 and Q2. | 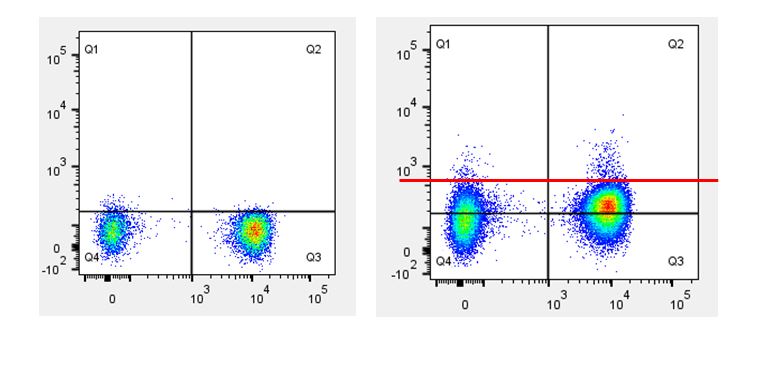 |
| Biological Controls |
| When staining with antibodies directed against cytokines or cell signaling molecules, the use of a biological control is a good choice. For example, if your samples include cells that have been stimulated with PMA & Ionomycin, LPS, or CD3/CD28, you should also include the same cell type that has not been stimulated. This is important for understanding the difference between the basal level of expression and any increase in expression due to upregulation caused by your stimulatory conditions. |
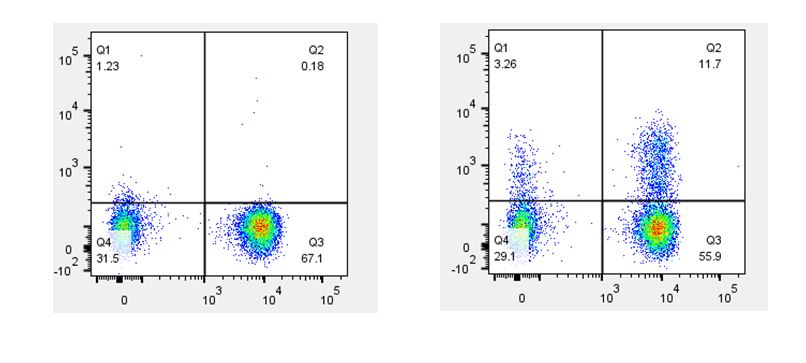 Th2 polarized human peripheral blood lymphocytes were treated with monensin alone (left) or treated with monensin and stimulated with PMA and Ionomycin (right) for three hours. Cells were then surface stained with CD3 (X axis) and intracellularly stained with GM-CSF (Y axis). |
| Fluorescence Minus One (FMO) Controls |
| Another important control to include in your intracellular staining experiment is a fluorescence minus one, or FMO. The FMO control consists of staining your samples with all but one of the conjugates in your panel. This control is not only useful when you have targets expressed at low levels, but also when it is difficult to distinguish between your negative and positive populations and to account for any spectral overlap you may encounter between fluorochromes in larger panels. |
| After reading this blog, we hope you have a good understanding of what is necessary when designing and performing an intracellular staining flow cytometry experiment. Choosing the optimal antibody concentration, correct buffer system, and best control(s) will ultimately lead you to generating consistent and reliable data. Should you have any additional questions regarding your intracellular staining experiment, do not hesitate to contact our tech support group at tech@biolegend.com. |
| Contributed by Kellie Johnson. |
 Login/Register
Login/Register 






Follow Us