Amyloid Precursor Protein (APP) is a type-I transmembrane protein with enriched expression in neuronal tissues. APP undergoes sequential proteolytic processing through two distinct pathways: 1) the amyloidogenic pathway that generates Amyloid Beta (Aβ) and 2) the non-amyloidogenic pathway that precludes the generation of Aβ. Aβ is generated when APP is cleaved by β-secretase, followed by cleavage from γ-secretase, while APP cleavage by α-secretase followed by γ-secretase does not generate Aβ peptide. The amyloidogenic pathway is associated with an accumulation of the neurotoxic Aβ peptide and is associated with Alzheimer's disease and other neurological diseases. BioLegend is proud to offer a wide range of high quality, well-characterized products to support research into the mechanism of neurological processes and its implications in neurodegenerative diseases.
Amyloid Precursor Protein and Aβ
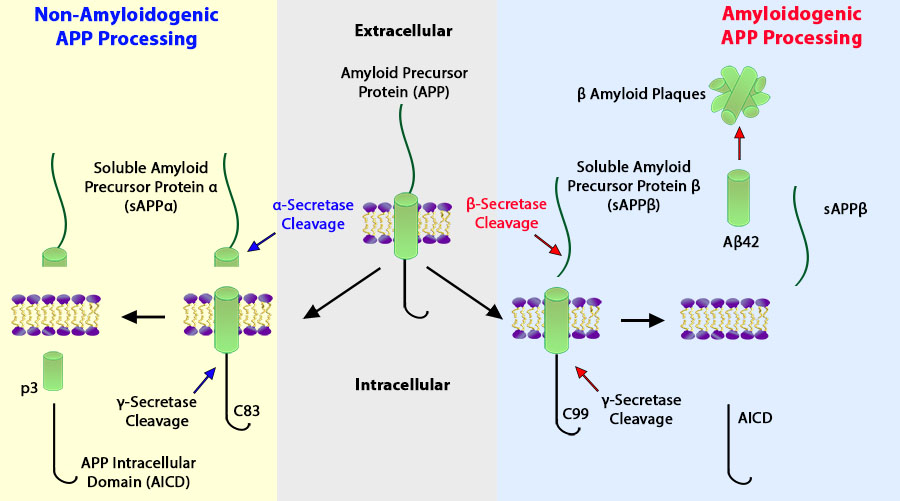
APP functions as a cell surface receptor and performs physiological functions on the surface of neurons relevant to neurite growth, neuronal adhesion and axonogenesis. It is involved in cell mobility and transcription regulation through protein-protein interactions.
Cleavage either by α-secretase, β-secretase or θ-secretase leads to generation and extracellular release of soluble APP peptides, sAPP-α and sAPP-β, and the retention of corresponding membrane-anchored C-terminal fragments, C80, C83 and C99. Subsequent processing of C80 and C83 by γ-secretase yields P3 peptides. This is the major secretory pathway and is non-amyloidogenic. Alternatively, presenilin/nicastrin-mediated γ-secretase processing of C99 releases the amyloid beta proteins, amyloid-beta 40 (Aβ40) and amyloid-beta 42 (Aβ42), major components of amyloid plaques, and the cytotoxic C-terminal fragments, gamma-CTF(50), gamma-CTF(57) and gamma-CTF(59). Many other minor β-amyloid peptides, β-amyloid 1-X peptides, are found in cerebral spinal fluid (CSF) including the β-amyloid X-15 peptides, produced from the cleavage by α-secretase and all terminating at Gln-686. It is proteolytically cleaved by caspases during neuronal apoptosis. Cleavage at Asp-739 by either caspase-6, -8, or -9 results in the production of the neurotoxic C31 peptide and the increased production of β-amyloid peptides.
|
Legend Green = Monoclonal Orange = Polyclonal Red = Monoclonal & Polyclonal Note: Diagrams are not to scale. |
BioLegend is proud to offer an extensive selection of high quality, well-characterized antibodies against APP and Aβ to support your neuroscience research. Mouse over the product name to view to protein segments identified by each antibody. Click on each name to view products.
Purified β-Amyloid, 1-15 Antibody (clone 3A1)
Clone 3A1 was generated using dityrosine cross-linked Aβ 1-40 as immunogen and demonstrates preference for aggregated Aβ. This antibody, unlike other gold standard antibodies for amyloid aggregate and plaque detection, such as clones 6E10 and 4G8, does not cross-reacts with amyloid precursor protein (APP). Clone 3A1 can be used in a variety of applications such as IHC, WB and ELISA.
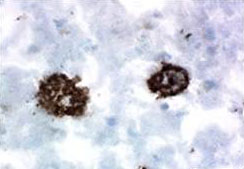
IHC staining of purified anti-β-Amyloid, 1-15 antibody (clone 3A1) on frozen Alzheimer's disease human brain tissue.
Purified anti-Beta Amyloid 1-42 (clone 12F4)
Amyloid beta (Aβ or Abeta) denotes peptides of 36–43 amino acids in length that are crucially involved in Alzheimer's disease as the main component of the amyloid plaques found in the brains of Alzheimer patients. The peptides result from the amyloid precursor protein (APP), which is cut by certain enzymes to yield Aβ. Aβ molecules can aggregate to form oligomers (known as "seeds") which are believed to be able to induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The seeds or the resulting amyloid plaques are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.
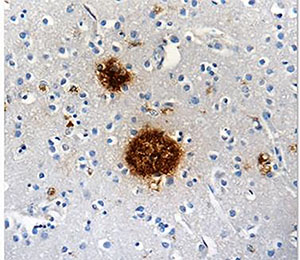
Staining of Clone B10AP on formalin-fixed paraffin-embedded Alzheimer's brain tissue.
Purified anti-β-Amyloid Pyroglutamyl (Glu3) (clone 337.48)
A large fraction of Aβ peptide in Alzheimer's Disease patients' hippocampus and cortex is cleaved to remove the first two amino acids. Subsequently, glutamate at the third residue position can be modified into pyroglutamate (pE). Aβ (pE3) tends to aggregate more easily and shows increased stability. This is potentially due to the loss of charged residues and the formation of a lactam ring, which leads to increased hydrophobicity, enhanced β-sheet formation, and resistance to peptidases. Others have shown that Aβ (pE3) is more toxic to neurons and astrocytes when compared to full length Aβ peptide. Like other Aβ peptides, Aβ (pE3) can form soluble oligomers. These oligomers can slip between synapses, preventing neurotransmitters from making contact with their receptors, causing synaptic failure and cell death. Oligomers can then join together to make amyloid plaques, which block cell communication and trigger inflammation.
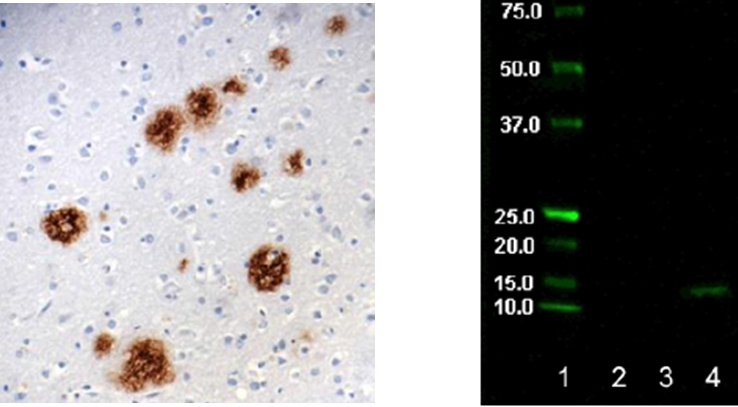
Left: Staining of pyroglutamyl E3 Amyloid Beta (clone 337.48) on formalin-fixed paraffin-embedded human Alzheimer's diseased brain tissue at 10µg/ml. Right: Western Blot showing specificity of Pyroglutamyl E3 Amyloid Beta. Lane 1:Molecular weight marker Lane 2: BSA neg ctrl Lane 3: Aβ1-40 peptide Lane 4: Hu Alz brain lysate (treated).
 Login/Register
Login/Register 






Follow Us