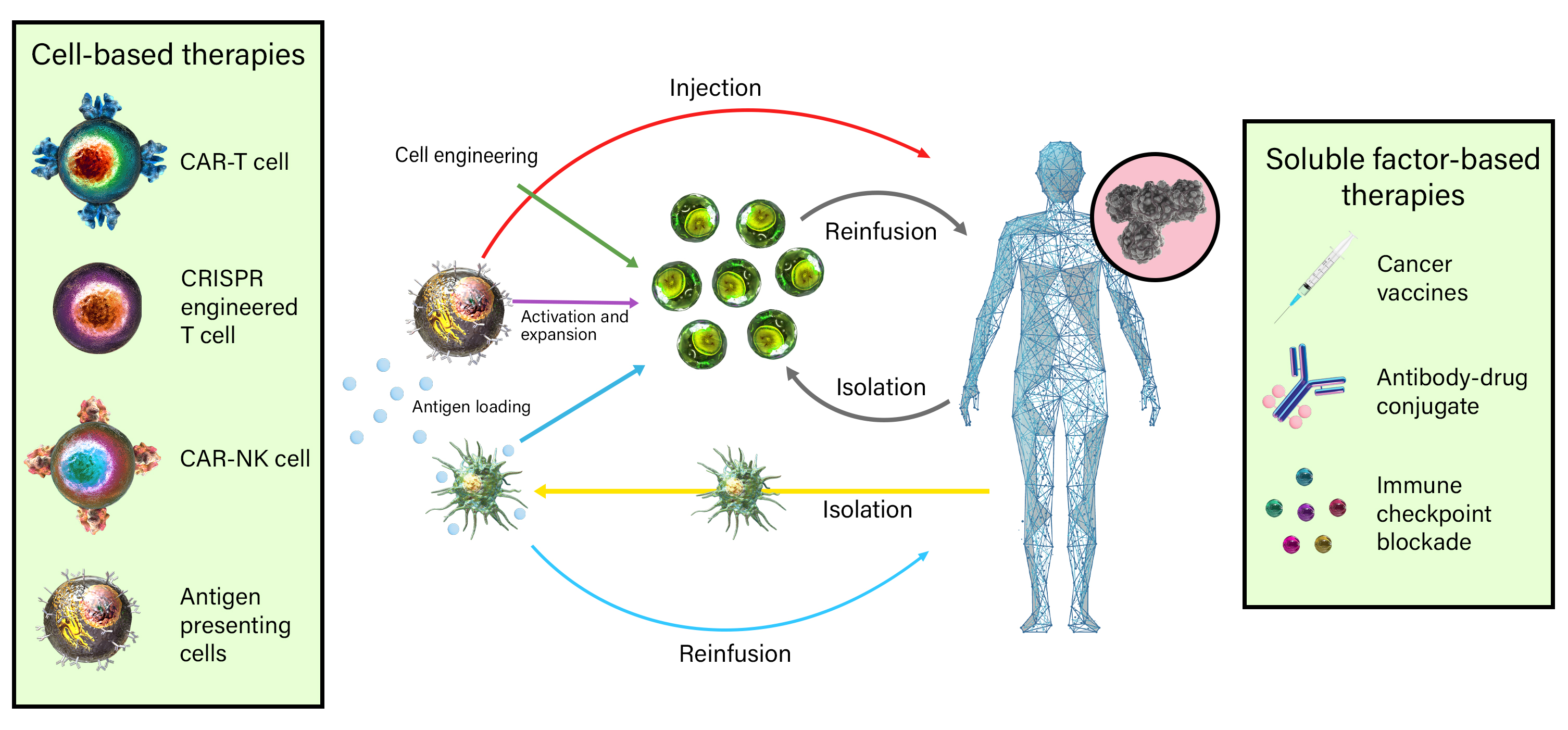
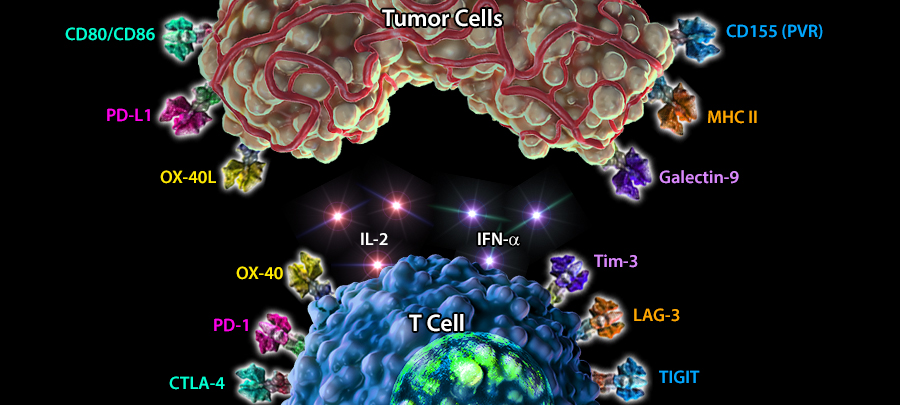
Immuno-oncology is a rapidly moving research area focused on stimulating or helping the immune system fight cancer. Immunotherapies are immune-based treatments for cancer. These include chimeric antigen receptor (CAR) T or Natural Killer (NK) cells engineered to recognize cancer cells, checkpoint inhibitors like anti-PD-1 and anti-CTLA-4 antibodies, cytokines like interleukins and interferons, and therapeutic vaccines that prime the immune system to attack cancer cells. Immunotherapies have shown clinical efficacy in treating cancers, spurring the growth of immunotherapy and immune-oncology research.
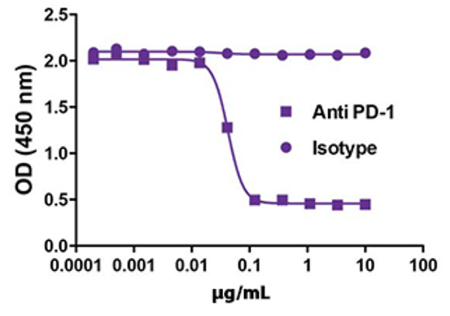
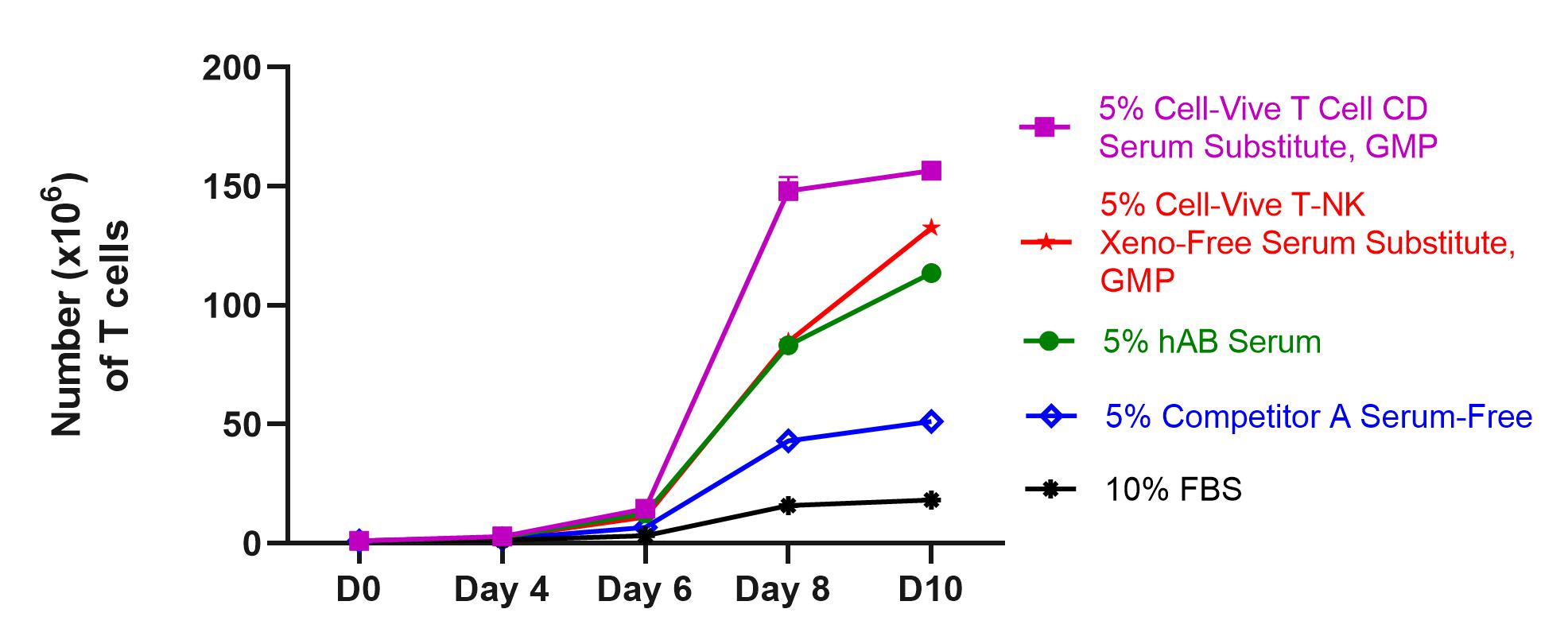
Study the roles of cytokines in combating tumor growth with highly stable recombinant proteins, and test strategies for stimulating anti-cancer immune responses with in vivo and ex vivo models with bioactive GoInVivo™ antibodies.
 Login / Register
Login / Register 







Follow Us