Tetramers - what are they and how can Flex-T™ help me with my research?
| Developed 20 years ago1, Major Histocompatibility Complex (MHC) tetramers have become widely available and easily accessible tools for the study and characterization of antigen-specific T cells. Tetramers consist of soluble MHC proteins complexed with a peptide, and are an efficient and easy way to identify, isolate, and study antigen-specific T cells from a variety of biological samples. T cells, or T lymphocytes, are important players in the cell-mediated immune response, and are normally characterized by the presence of cell surface proteins such as CD4 or CD82. When exposed to certain stimuli, the T cells differentiate into particular subsets. One of these stimuli is an antigenic peptide that is presented to the T cell mostly by two classes of MHC. All nucleated cells have MHC-I molecules, while MHC-II molecules are usually only found on Antigen Presenting Cells (APCs). We will only focus on class I and II for this blog, as they are the ones that mainly present peptides to T cells. The peptide-MHC complexes are recognized by the T Cell Receptor (TCR) and the CD co-receptor (CD4 or CD8). In order for the T cells to become activated, both the TCR and the CD co-receptor must bind to the peptide-MHC complex. The specificity of binding is conferred through the TCR; TCRs are specific to certain peptide sequences and therefore each T cell will recognize a unique peptide sequence that is associated with a particular pathogen. In a healthy person with a normal immune system, any pathogen that they have encountered in their lifetime will have activated a T cell with a TCR that is specific to a peptide that is present on that pathogen. These T cell clones then multiply in order to help get rid of the pathogen. |
| One property of the peptide-MHC complex that has made studying it so difficult is its low affinity for the TCR. This is necessary in order to allow each TCR molecule to bind multiple MHC-peptide complexes, and is the reason for the development of tetramers. When multimerized, the MHC complex/TCR interaction becomes more stable as the tetramer can bind multiple TCRs on the cell surface, in turn increasing the TCR binding affinity and decreasing the dissociation rate. So what exactly is a tetramer and how is it made? |
| At the heart of the tetramer is a fluorescently labeled streptavidin molecule which can bind four biotin molecules3. MHC tetramers are made of 4 biotinylated MHC complexes, loaded with the peptide of interest (8-10 amino acids for MHC-I; 14-20 amino acids for MHC-II)4. There are multiple ways to make these proteins – the most common is the enzymatic method. The heavy chain, the β2-microglobulin invariant light chain, the peptide, and the enzyme BirA are co-expressed in Escherichia coli and mixed together with the peptide. There is a 15 amino acid recognition tag expressed on the C-terminus of the MHC α-chain that allows the BirA enzyme to specifically biotinylate one lysine residue within the recognition tag. The monomers are then purified by size- and charge-exclusion chromatography, and the multimerization step happens upon the addition of streptavidin3. Since one streptavidin molecule can bind four biotins, the result is a tetramer. It is also possible to have multimers, depending on how many biotins the backbone of the multimer can bind. |
| If you are interested in a specific peptide, or want to use many different peptides, it’s possible to make the tetramers using an ultraviolet light photolabile peptide that can be displaced by the peptide of interest. This method allows for large-scale screening of any peptide of interest. BioLegend’s Flex-T™ MHC Tetramers offer the flexibility (see what we did there?) of loading any compatible peptide into the binding site of the complex, providing an affordable, easy way of studying antigen-specific T cells. You can learn more about the compatible antigen peptides here. If your peptide of interest is not listed, we also offer custom Flex-T™ monomer or tetramers – request yours here. |
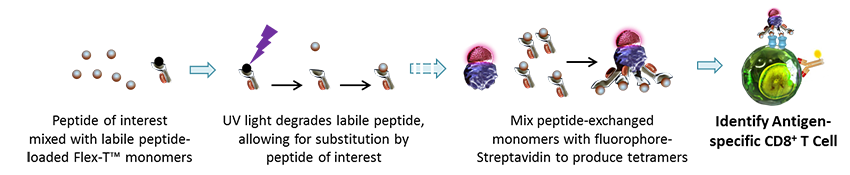 |
| Now that you understand how the tetramers are made, you can probably think of many ways where this technology could be useful – and you would be right! Tetramers/multimers have been used by researchers to identify and quantify antigen-specific T cells from patient samples, which is especially useful when determining the effectiveness of a vaccine3. The frequency of CD8+ T cells can be assessed by enriching the sample for CD8+ T cells using a magnetic bead protocol, followed by staining with tetramers to identify a specific population and track the changes in that population over time. This can help understand disease progression as well as vaccine response, and can help inform doctors of how well the patient is responding to therapeutic intervention. Different tetramers can also be labeled with different fluorophores, allowing for a multicolor analysis to track multiple specificities of T cells from the same patient sample. |
 |
 |
| Possible combinations of two-color codes depend on the number of Streptavidin-fluorophore conjugates used to generate the tetramer/peptide complex. |
| It’s also possible to use tetramers to guide epitope mapping, more commonly used with MHC class II tetramers5. Instead of loading the MHC molecules with a specific peptide, they are loaded with a mixture of peptides from overlapping regions of the antigen of interest. These are then divided into pools of five to ten peptides and used to analyze cells that were stimulated with the antigen of interest. Once there is a positive stain, the individual peptides can then be loaded to identify T cells with a particular antigen specificity and MHC restriction. These cells can then be expanded in vitro and transferred back into the patient, as has been done for the treatment of HIV and cytomegalovirus (CMV) infections. All nine patients who received transplants of CMV-specific T cells showed a reduction in CMV viremia, with no observable side effects6. One patient had a chronic CMV infection that was refractory to treatment with antivirals but was controlled within 8 days of receiving the CMV-specific T cell transplant. It is important to be able to control CMV infection in immunocompromised patients, such as those with active HIV infection or cancer patients. Dextramers were used to identify melanoma-specific CD8+ T cells from a solid tumor and from peripheral blood7. This study established the use of multimers in samples other than peripheral blood, and there are more similar studies underway across the world. |
| Tetramer assays have expanded the toolbox of researchers and physicians, allowing them to not only learn more about antigen-specific T cells, but about the immune response to pathogens and cancer in general. There is much more to learn about the immune system and how it interacts and responds to attack by foreign and self-associated antigens. Tetramers will continue to aid such discoveries and help to drive disease treatments in the future. Do you work with tetramers or have research you want to share with us? Get in touch with the Tech Team here! |
 Login / Register
Login / Register 






Follow Us