Microfilaments
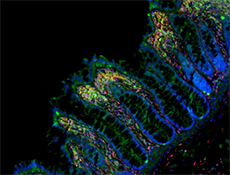
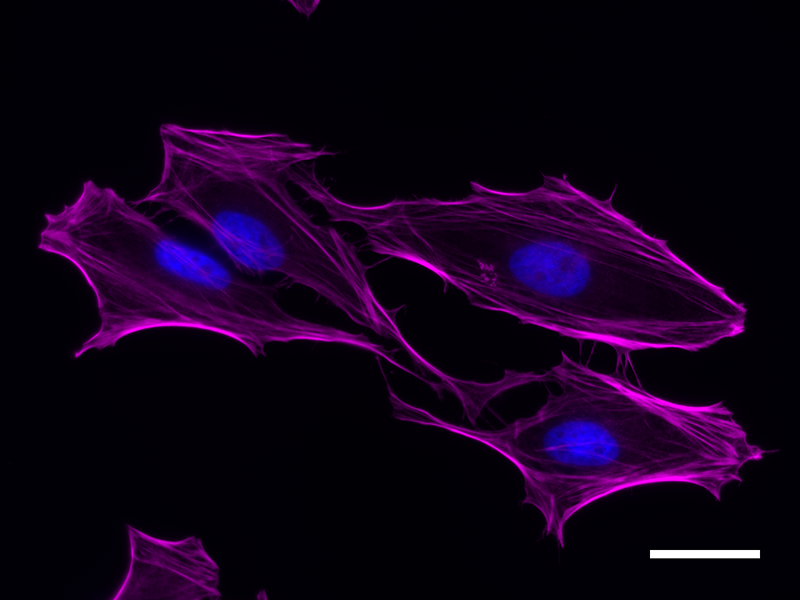
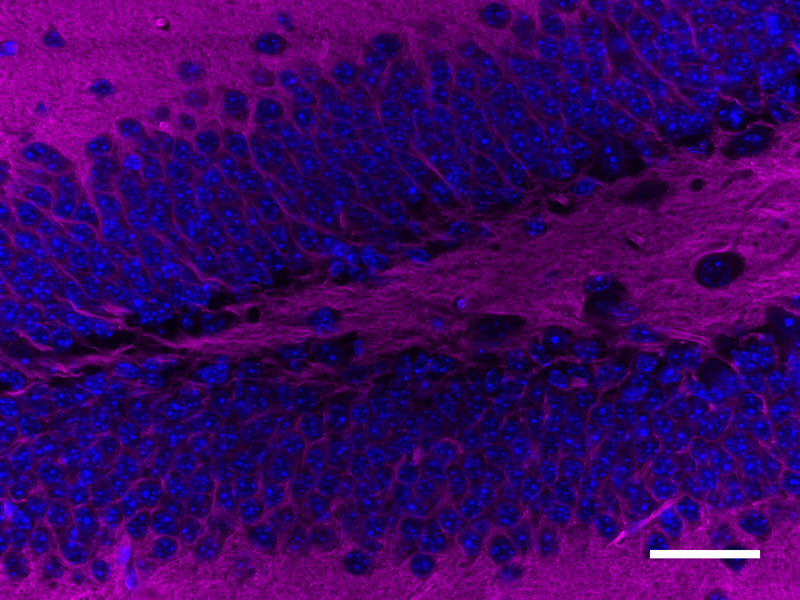
Microfilaments are protein filaments composed of actin polymers that function in cell contractility, cell motility, cytokinesis, endocytosis, and exocytosis.
Actin is an ATPase that is abundantly expressed throughout the cytoplasm and in the nucleus. Actin filament polymerization and depolymerization is a highly dynamic and tightly regulated process that is dependent on the function of accessory and regulatory proteins. Polymerization occurs at the ‘barbed’ end, while depolymerization takes place at the opposite, pointed filament end1. The terms G- and F-actin refer to actin in a free monomeric state and actin incorporated into filaments, respectively.
Phalloidin is a bicyclic peptide found in death cap mushrooms that binds very tightly to F-actin, preventing its depolymerization in living cells. In cellular imaging, fluorescently conjugated phalloidins are useful for imaging the fine filaments of actin, providing structural and volumetric context to the cell. We provide Flash Phalloidin™ formats that emit in the green (488 nm) and red (594 nm) regions.
 Login / Register
Login / Register 






Follow Us