Cell-Vive™ Replaces Human Serum and Optimally Cultures T and NK Cells

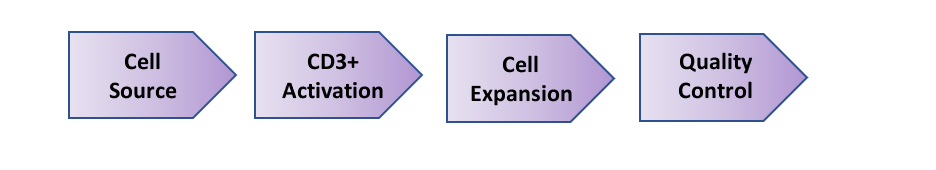
Cell-Vive™️ T-NK Xeno-Free Serum Substitute, GMP is specifically formulated to proliferate T cells and Natural Killer (NK) cells for cell therapy manufacturing. Among the most promising cancer treatments is cellular immunotherapy, which can involve the use of a patient’s own T and NK cells, to target and eliminate cancer. Source cells, such as peripheral blood mononuclear cells (PBMCs), are isolated from a patient’s blood. T cells are then activated using anti-CD3/CD28 antibodies and cytokines, followed by cell culturing and expansion. After expansion, grown T cells undergo quality control to ensure they match the necessary requirements, such as cell identity and high yields needed for immunotherapy (Fig 1). Finally, cells are then infused back into the patient as therapy, or cells are cryopreserved for later use at a treatment facility.
Figure 1. T Cell Bioprocessing Workflow
Disadvantages of Human Serum
Within this process, activating and expanding anti-tumor T and NK cells are critical early steps. For immune cell culturing and expansion, human AB serum has traditionally been added to supplement culturing media. As a supplement, human serum provides an important, complex mix of vital nutrients and other factors that enhance survival, activation, and growth of cells. Use of human serum, however, comes with unwanted costs and risks. Along with nutrients, serum includes undefined components, nitrogenous waste products, and could be influenced by blood donor-specific characteristics such as gender, age, diet, and underlying health issues. Serum also comes with risks to end-users due to potential for pathogens. In addition, the use of serum is costly due to inherent lot-to-lot inconsistencies, resulting in additional time and labor costs to end-users having to test and qualify each lot to ensure performance.
Serum-Free Culturing: Increased Safety, Consistency, and Performance
Cell-Vive™️ T-NK Xeno-Free Serum Substitute, GMP is a defined media reagent that replaces human serum for cell culturing, thereby avoiding safety risks and lot-to-lot inconsistencies. It is precisely formulated to optimally grow cells while providing researchers more control over cell culture conditions. Furthermore, our serum substitute comes with full protocols optimized for the culturing and expansion of T cells and NK cells.
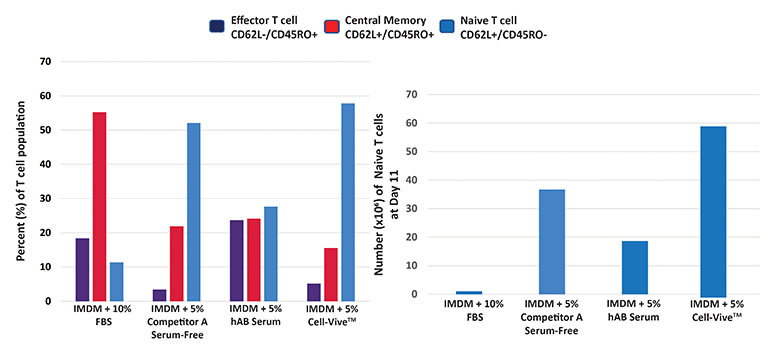
Using a typical protocol for T cell culturing and expansion, Ultra-LEAF™ anti-human CD3 and Ultra-LEAF™ anti-human CD28 antibodies were coated onto plates along with complete media. In this display experiment, complete media consists of basal media like IMDM, supplemented with either 10% FBS, 5% v/v human serum, a competitor serum-replacement reagent, or Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP and 200 IU/mL recombinant human IL-2 cytokine. PBMCs are then added to coated plates and activated, yielding PBMC-derived T cells. As the data shows, Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP supports greater cell expansion of naïve T cells (left), generating a higher number of naïve T cells per million of PBMCs plated by day 11 (right).
For NK cell expansion, PBMC-derived NK cells were cultured in basal media supplemented with the same aforementioned culture additives, 1000 IU/mL recombinant human IL-2 cytokine, along with inactivated K562 feeder cells. As the data shows, Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP also promotes greater NK cell expansion.
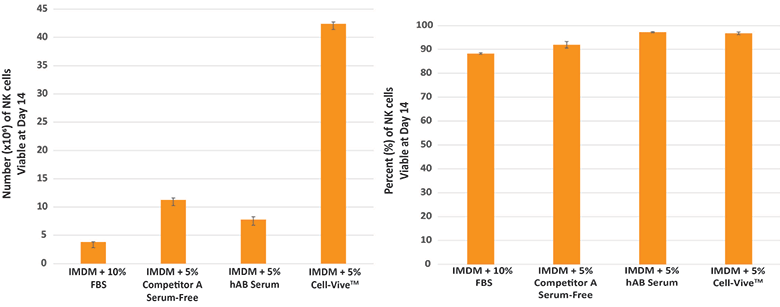
The greater yield, in the case of both T and NK cells is a critical, desired feature for cell therapies.
Applications: GMP Relates to Cell Therapy and Cell Bioprocessing
Compliance with governmental regulations is necessary to produce cell therapies. Enforced by the FDA in the USA, Good Manufacturing Practice (GMP) is the framework that ensures products are consistently produced and controlled according to quality requirements. GMP products are manufactured with quality documentation and full traceability, under independent QA oversight. cGMP (current GMP) means that the manufacturer is following the most updated standards. All BioLegend GMP products are manufactured in a dedicated GMP facility and are compliant with our ISO 13485:2016-certified quality management system. Our GMP products are manufactured and tested in accordance with USP Chapter 1043, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products Ph. Eur. Chapter 5.2.12. BioLegend GMP products are not intended for direct therapeutic or diagnostic purposes. In addition, each lot of Cell-Vive™ T-NK Xeno-Free Serum Substitute is QC tested for endotoxin, pH, osmolality, Mycoplasma, sterility, and T cell function validation.
Cell therapy strategies have made personalized and novel therapeutics possible, certainly revolutionizing cancer treatment. A critical stage in such strategies is the culturing and expansion of T and NK cells. However, serum batches vary in composition, thereby affecting numbers and types of immune cells produced, and comes with added cost and risks. We formulated Cell-Vive™ T-NK Xeno-Free Serum Substitute to specifically culture and expand T cells and NK cells, adding the GMP seal to ensure suitability for research or cell bioprocessing.
For more information, visit BioLegend GMP Reagents.
This article originally appeared on Cell Culture Dish.
 Login / Register
Login / Register 






Follow Us