Choose the Correct Fc Receptor Block for Cell Phenotyping Procedures
Flow cytometry is a precise method to phenotype different cell types within a heterogenous sample using fluorescently tagged antibodies as probes to recognize cell surface or intracellular markers. Flow cytometry (FC) is now an indispensable tool in clinical applications such as tracking a patient’s response to treatment and diagnosing and monitoring immune disorders, cancers, and infectious diseases.
Achieving accurate cell analysis results hinges on experimental design. Undesirable binding between the Fc portion of antibodies and the Fc receptors (FcR) found on many types of immune cells can lead to false positives and meaningless data1. To ensure purity of enriched cells, cell separation and/or staining procedures should include FcR blockers. Therefore, the choice of FcR blocker can significantly impact the accuracy and reliability of flow cytometry assay results.
In this blog, we walk you through the choice between two high quality FcR blockers, Human TruStain FcX™, GMP and Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP, and help unlock the precision in your flow cytometry assays.
|
Human TruStain FcX™, GMP |
Cell-Vive™ Ultra-LEAF Defined Human TruStain FcX™, GMP |
|
Both are specially formulated for blocking unwanted FcR-related staining without interfering with antibody-mediated specific staining of human cells. |
|
|
Both are compatible with flow cytometric staining with anti-human CD16 (clone 3G8), CD32 (clone FUN-2), and CD64 (clone 10.1) antibodies. |
|
|
Formulated as human IgG in PBS with 0.09% sodium azide. |
Formulated as serum-free, defined, with ultra-low endotoxin levels (<0.01EU/µg of protein), and is tested negative for Mycoplasma and microbial growths. |
|
Ideal for applications requiring GMP-grade cell characterization for quality control and immune-monitoring. |
Suited for GMP-grade cell isolation and enrichment, as well as characterization applications that demand stringent manufacturing processes, especially concerning endotoxin levels and microbial contamination. |
|
Ideal for R&D laboratories requiring a reliable FcR blocker in recurring FC experiments. |
Tailored for cell bioprocessing laboratories and those transitioning to ex vivo bioprocessing applications. Suitable to be used as an ancillary reagent. Tested according to USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Ph. Eur. Chapter 5.2.12 |
|
The formulation you choose will depend on your intended application, experimental goals, and regulatory compliance requirements. THP-1 cells were treated with Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP followed by staining with an irrelevant PE mouse IgG2a, κ Isotype (clone MOPC-173, purple histogram). Non-specific binding of the same antibody on untreated cells is shown in red. 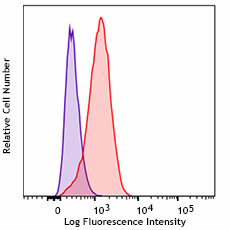 |
|
In summary, BioLegend offers Human TruStain FcX™, GMP, and Cell-Vive™ Ultra-LEAF™ Defined Human TruStain FcX™, GMP, both of which block Fc receptors and reduce non-specific immunofluorescent staining. We give you a choice of formulations that can be applied to the specific needs of your experiments.

References
- Andersen, Morten N et al. “Elimination of erroneous results in flow cytometry caused by antibody binding to Fc receptors on human monocytes and macrophages.” Cytometry. Part A : the journal of the International Society for Analytical Cytology vol. 89,11 (2016): 1001-1009. doi:10.1002/cyto.a.22995. PubMed.
 Login / Register
Login / Register 






Follow Us